Simultaneous cortex-wide fluorescence Ca2+ imaging and whole-brain fMRI
- PMID: 33139894
- PMCID: PMC7704940
- DOI: 10.1038/s41592-020-00984-6
Simultaneous cortex-wide fluorescence Ca2+ imaging and whole-brain fMRI
Erratum in
-
Author Correction: Simultaneous cortex-wide fluorescence Ca2+ imaging and whole-brain fMRI.Nat Methods. 2021 Jul;18(7):835. doi: 10.1038/s41592-021-01217-0. Nat Methods. 2021. PMID: 34163088 No abstract available.
Abstract
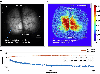
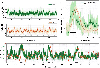
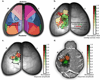
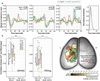
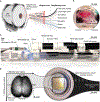
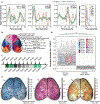
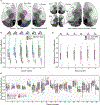
Achieving a comprehensive understanding of brain function requires multiple imaging modalities with complementary strengths. We present an approach for concurrent widefield optical and functional magnetic resonance imaging. By merging these modalities, we can simultaneously acquire whole-brain blood-oxygen-level-dependent (BOLD) and whole-cortex calcium-sensitive fluorescent measures of brain activity. In a transgenic murine model, we show that calcium predicts the BOLD signal, using a model that optimizes a gamma-variant transfer function. We find consistent predictions across the cortex, which are best at low frequency (0.009-0.08 Hz). Furthermore, we show that the relationship between modality connectivity strengths varies by region. Our approach links cell-type-specific optical measurements of activity to the most widely used method for assessing human brain function.
Figures









Similar articles
-
Macroscale variation in resting-state neuronal activity and connectivity assessed by simultaneous calcium imaging, hemodynamic imaging and electrophysiology.Neuroimage. 2018 Apr 1;169:352-362. doi: 10.1016/j.neuroimage.2017.12.070. Epub 2017 Dec 22. Neuroimage. 2018. PMID: 29277650 Free PMC article.
-
Fiber-optic implant for simultaneous fluorescence-based calcium recordings and BOLD fMRI in mice.Nat Protoc. 2018 May;13(5):840-855. doi: 10.1038/nprot.2018.003. Epub 2018 Mar 29. Nat Protoc. 2018. PMID: 29599439
-
Resting state brain function analysis using concurrent BOLD in ASL perfusion fMRI.PLoS One. 2013 Jun 4;8(6):e65884. doi: 10.1371/journal.pone.0065884. Print 2013. PLoS One. 2013. PMID: 23750275 Free PMC article.
-
The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal.Philos Trans R Soc Lond B Biol Sci. 2002 Aug 29;357(1424):1003-37. doi: 10.1098/rstb.2002.1114. Philos Trans R Soc Lond B Biol Sci. 2002. PMID: 12217171 Free PMC article. Review.
-
Multiparametric measurement of cerebral physiology using calibrated fMRI.Neuroimage. 2019 Feb 15;187:128-144. doi: 10.1016/j.neuroimage.2017.12.049. Epub 2017 Dec 19. Neuroimage. 2019. PMID: 29277404 Review.
Cited by
-
Fiber-based lactate recordings with fluorescence resonance energy transfer sensors by applying an magnetic resonance-informed correction of hemodynamic artifacts.Neurophotonics. 2022 Jul;9(3):032212. doi: 10.1117/1.NPh.9.3.032212. Epub 2022 May 9. Neurophotonics. 2022. PMID: 35558647 Free PMC article.
-
Study of neurovascular coupling by using mesoscopic and microscopic imaging.iScience. 2021 Sep 25;24(10):103176. doi: 10.1016/j.isci.2021.103176. eCollection 2021 Oct 22. iScience. 2021. PMID: 34693226 Free PMC article.
-
Wide-field calcium imaging of cortex-wide activity in awake, head-fixed mice.STAR Protoc. 2021 Nov 20;2(4):100973. doi: 10.1016/j.xpro.2021.100973. eCollection 2021 Dec 17. STAR Protoc. 2021. PMID: 34849490 Free PMC article.
-
Diverse and asymmetric patterns of single-neuron projectome in regulating interhemispheric connectivity.Nat Commun. 2024 Apr 22;15(1):3403. doi: 10.1038/s41467-024-47762-y. Nat Commun. 2024. PMID: 38649683 Free PMC article.
-
Network models to enhance the translational impact of cross-species studies.Nat Rev Neurosci. 2023 Sep;24(9):575-588. doi: 10.1038/s41583-023-00720-x. Epub 2023 Jul 31. Nat Rev Neurosci. 2023. PMID: 37524935 Free PMC article. Review.
References
-
- Albers F et al. Multimodal functional neuroimaging by simultaneous BOLD fMRI and fiber-optic calcium recordings and optogenetic control. Mol Imaging Biol, 20, 171–182 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

