Beyond Shielding: The Roles of Glycans in the SARS-CoV-2 Spike Protein
- PMID: 33140034
- PMCID: PMC7523240
- DOI: 10.1021/acscentsci.0c01056
Beyond Shielding: The Roles of Glycans in the SARS-CoV-2 Spike Protein
Abstract
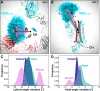
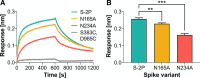
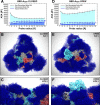
The ongoing COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in more than 28,000,000 infections and 900,000 deaths worldwide to date. Antibody development efforts mainly revolve around the extensively glycosylated SARS-CoV-2 spike (S) protein, which mediates host cell entry by binding to the angiotensin-converting enzyme 2 (ACE2). Similar to many other viral fusion proteins, the SARS-CoV-2 spike utilizes a glycan shield to thwart the host immune response. Here, we built a full-length model of the glycosylated SARS-CoV-2 S protein, both in the open and closed states, augmenting the available structural and biological data. Multiple microsecond-long, all-atom molecular dynamics simulations were used to provide an atomistic perspective on the roles of glycans and on the protein structure and dynamics. We reveal an essential structural role of N-glycans at sites N165 and N234 in modulating the conformational dynamics of the spike's receptor binding domain (RBD), which is responsible for ACE2 recognition. This finding is corroborated by biolayer interferometry experiments, which show that deletion of these glycans through N165A and N234A mutations significantly reduces binding to ACE2 as a result of the RBD conformational shift toward the "down" state. Additionally, end-to-end accessibility analyses outline a complete overview of the vulnerabilities of the glycan shield of the SARS-CoV-2 S protein, which may be exploited in the therapeutic efforts targeting this molecular machine. Overall, this work presents hitherto unseen functional and structural insights into the SARS-CoV-2 S protein and its glycan coat, providing a strategy to control the conformational plasticity of the RBD that could be harnessed for vaccine development.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Update of
-
Beyond Shielding: The Roles of Glycans in SARS-CoV-2 Spike Protein.bioRxiv [Preprint]. 2020 Sep 4:2020.06.11.146522. doi: 10.1101/2020.06.11.146522. bioRxiv. 2020. Update in: ACS Cent Sci. 2020 Oct 28;6(10):1722-1734. doi: 10.1021/acscentsci.0c01056. PMID: 32577644 Free PMC article. Updated. Preprint.
References
-
- Chan J. F. W.; Yuan S.; Kok K. H.; To K. K. W.; Chu H.; Yang J.; Xing F.; Liu J.; Yip C. C. Y.; Poon R. W. S.; Tsoi H. W.; Lo S. K. F.; Chan K. H.; Poon V. K. M.; Chan W. M.; Ip J. D.; Cai J. P.; Cheng V. C. C.; Chen H.; Hui C. K. M.; Yuen K. Y. A Familial Cluster of Pneumonia Associated with the 2019 Novel Coronavirus Indicating Person-to-Person Transmission: A Study of a Family Cluster. Lancet 2020, 395 (10223), 514–523. 10.1016/S0140-6736(20)30154-9. - DOI - PMC - PubMed
-
- Chan J. F.-W.; Kok K.-H.; Zhu Z.; Chu H.; To K. K.-W.; Yuan S.; Yuen K.-Y. Genomic Characterization of the 2019 Novel Human-Pathogenic Coronavirus Isolated from a Patient with Atypical Pneumonia after Visiting Wuhan. Emerging Microbes Infect. 2020, 9 (1), 221–236. 10.1080/22221751.2020.1719902. - DOI - PMC - PubMed
-
- Remembering the Nearly 100,000 Lives Lost to Coronavirus in America - The New York Times. https://www.nytimes.com/interactive/2020/05/24/us/us-coronavirus-deaths-... (accessed May 25, 2020).
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

