This is a preprint.
Saliva is a promising alternative specimen for the detection of SARS-CoV-2 in children and adults
- PMID: 33140064
- PMCID: PMC7605576
- DOI: 10.1101/2020.10.25.20219055
Saliva is a promising alternative specimen for the detection of SARS-CoV-2 in children and adults
Update in
-
Saliva Is a Promising Alternative Specimen for the Detection of SARS-CoV-2 in Children and Adults.J Clin Microbiol. 2021 Jan 21;59(2):e02686-20. doi: 10.1128/JCM.02686-20. Print 2021 Jan 21. J Clin Microbiol. 2021. PMID: 33239380 Free PMC article.
Abstract
Testing efforts for SARS-CoV-2 have been burdened by the scarcity of testing materials and personal protective equipment for healthcare workers. The simple and painless process of saliva collection allows for widespread testing, but enthusiasm is hampered by variable performance compared to nasopharyngeal swab (NPS) samples. We prospectively collected paired NPS and saliva samples from a total of 300 unique adult and pediatric patients. SARS-CoV-2 RNA was detected in 32.2% (97/300) of the individuals using the TaqPath COVID-19 Combo Kit (Thermo Fisher). Performance of saliva and NPS were compared against the total number of positives regardless of specimen type. The overall concordance for saliva and NPS was 91.0% (273/300) and 94.7% (284/300), respectively. The positive percent agreement (PPA) for saliva and NPS was 81.4% (79/97) and 89.7% (87/97), respectively. Saliva detected 10 positive cases that were negative by NPS. In symptomatic and asymptomatic pediatric patients not previously diagnosed with COVID-19, the performances of saliva and NPS were comparable (PPA: 82.4% vs 85.3%). The overall PPA for adults were 83.3% and 90.7% for saliva and NPS, respectively, with saliva detecting 4 cases less than NPS. However, saliva performance in symptomatic adults was identical to NPS (PPA of 93.8%). With lower cost and self-collection capabilities, saliva can be an appropriate alternative sample choice to NPS for detection of SARS-CoV-2 in children and adults.
Summary: Saliva is an acceptable alternative specimen compared to nasopharyngeal swabs for detection of SARS-CoV-2. Specifically, saliva demonstrated comparable performance to nasopharyngeal swabs in symptomatic and asymptomatic pediatric patients and in symptomatic adults.
Conflict of interest statement
Conflict of Interest
All authors declare no conflict of interest.
Figures


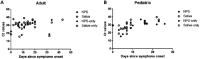
References
-
- Hanson KE, Caliendo AM, Arias CA, Englund JA, Lee MJ, Loeb M, Patel R, El Alayli A, Kalot MA, Falck-Ytter Y, Lavergne V, Morgan RL, Murad MH, Sultan S, Bhimraj A, Mustafa RA. 2020. Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19. Clin Infect Dis doi: 10.1093/cid/ciaa760 - DOI - PMC - PubMed
-
- Ranney ML, Griffeth V, Jha AK. 2020. Critical Supply Shortages - The Need for Ventilators and Personal Protective Equipment during the Covid-19 Pandemic. N Engl J Med 382:e41. - PubMed
-
- Sueki A, Matsuda K, Yamaguchi A, Uehara M, Sugano M, Uehara T, Honda T. 2016. Evaluation of saliva as diagnostic materials for influenza virus infection by PCR-based assays. Clin Chim Acta 453:71–4. - PubMed
-
- To KKW, Yip CCY, Lai CYW, Wong CKH, Ho DTY, Pang PKP, Ng ACK, Leung KH, Poon RWS, Chan KH, Cheng VCC, Hung IFN, Yuen KY. 2019. Saliva as a diagnostic specimen for testing respiratory virus by a point-of-care molecular assay: a diagnostic validity study. Clin Microbiol Infect 25:372–378. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
