Longitudinal Serological Analysis and Neutralizing Antibody Levels in Coronavirus Disease 2019 Convalescent Patients
- PMID: 33140086
- PMCID: PMC7665595
- DOI: 10.1093/infdis/jiaa659
Longitudinal Serological Analysis and Neutralizing Antibody Levels in Coronavirus Disease 2019 Convalescent Patients
Abstract
Background: Understanding the longitudinal trajectory of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies is crucial for diagnosis of prior infection and predicting future immunity.
Methods: We conducted a longitudinal analysis of coronavirus disease 2019 convalescent patients, with neutralizing antibody assays and SARS-CoV-2 serological assay platforms using SARS-CoV-2 spike (S) or nucleocapsid (N) antigens.
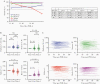
Results: Sensitivities of serological assays in diagnosing prior SARS-CoV-2 infection changed with time. One widely used commercial platform that had an initial sensitivity of >95% declined to 71% at 81-100 days after diagnosis. The trajectories of median binding antibody titers measured over approximately 3-4 months were not dependent on the use of SARS-CoV-2 N or S proteins as antigen. The median neutralization titer decreased by approximately 45% per month. Each serological assay gave quantitative antibody titers that were correlated with SARS-CoV-2 neutralization titers, but S-based serological assay measurements better predicted neutralization potency. Correlation between S-binding and neutralization titers deteriorated with time, and decreases in neutralization titers were not predicted by changes in S-binding antibody titers.
Conclusions: Different SARS-CoV-2 serological assays are more or less well suited for surveillance versus prediction of serum neutralization potency. Extended follow-up should facilitate the establishment of appropriate serological correlates of protection against SARS-CoV-2 reinfection.
Keywords: COVID-19; Neutralizing antibodies; SARS-CoV-2; Serology.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures


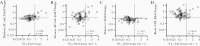
Update of
-
Longitudinal analysis of clinical serology assay performance and neutralising antibody levels in COVID19 convalescents.medRxiv [Preprint]. 2020 Aug 6:2020.08.05.20169128. doi: 10.1101/2020.08.05.20169128. medRxiv. 2020. Update in: J Infect Dis. 2021 Feb 13;223(3):389-398. doi: 10.1093/infdis/jiaa659. PMID: 32793928 Free PMC article. Updated. Preprint.
Comment in
-
Longitudinal Serological Analysis Following a National Seroprevalence Study to Investigate COVID-19 Infection in People Living in Ireland.J Infect Dis. 2021 Sep 17;224(6):1100-1101. doi: 10.1093/infdis/jiab346. J Infect Dis. 2021. PMID: 34192334 Free PMC article. No abstract available.
References
-
- Andersson M, Low N, French N, et al. Rapid roll out of SARS-CoV-2 antibody testing—a concern. BMJ 2020; 369:m2420. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

