PTRF/cavin-1 remodels phospholipid metabolism to promote tumor proliferation and suppress immune responses in glioblastoma by stabilizing cPLA2
- PMID: 33140095
- PMCID: PMC7992898
- DOI: 10.1093/neuonc/noaa255
PTRF/cavin-1 remodels phospholipid metabolism to promote tumor proliferation and suppress immune responses in glioblastoma by stabilizing cPLA2
Abstract
Background: Metabolism remodeling is a hallmark of glioblastoma (GBM) that regulates tumor proliferation and the immune microenvironment. Previous studies have reported that increased polymerase 1 and transcript release factor (PTRF) levels are associated with a worse prognosis in glioma patients. However, the biological role and the molecular mechanism of PTRF in GBM metabolism remain unclear.
Methods: The relationship between PTRF and lipid metabolism in GBM was detected by nontargeted metabolomics profiling and subsequent lipidomics analysis. Western blotting, quantitative real-time PCR, and immunoprecipitation were conducted to explore the molecular mechanism of PTRF in lipid metabolism. A sequence of in vitro and in vivo experiments (both xenograft tumor and intracranial tumor mouse models) were used to detect the tumor-specific impacts of PTRF.
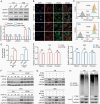
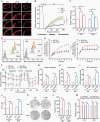
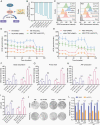
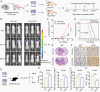
Results: Here, we show that PTRF triggers a cytoplasmic phospholipase A2 (cPLA2)-mediated phospholipid remodeling pathway that promotes GBM tumor proliferation and suppresses tumor immune responses. Research in primary cell lines from GBM patients revealed that cells overexpressing PTRF show increased cPLA2 activity-resulting from increased protein stability-and exhibit remodeled phospholipid composition. Subsequent experiments revealed that PTRF overexpression alters the endocytosis capacity and energy metabolism of GBM cells. Finally, in GBM xenograft and intracranial tumor mouse models, we showed that inhibiting cPLA2 activity blocks tumor proliferation and prevents PTRF-induced reduction in CD8+ tumor-infiltrating lymphocytes.
Conclusions: The PTRF-cPLA2 lipid remodeling pathway promotes tumor proliferation and suppresses immune responses in GBM. In addition, our findings highlight multiple new therapeutic targets for GBM.
Keywords: PTRF; cPLA2; energy metabolism; glioblastoma; phospholipid remodeling.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


Comment in
-
Targeting phospholipid metabolism for glioblastoma therapy.Neuro Oncol. 2021 Mar 25;23(3):343-344. doi: 10.1093/neuonc/noaa309. Neuro Oncol. 2021. PMID: 33560441 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

