A New Intraepithelial γδ T-Lymphocyte Marker for Celiac Disease Classification in Formalin-Fixed Paraffin-Embedded (FFPE) Duodenal Biopsies
- PMID: 33140183
- PMCID: PMC8449760
- DOI: 10.1007/s10620-020-06680-x
A New Intraepithelial γδ T-Lymphocyte Marker for Celiac Disease Classification in Formalin-Fixed Paraffin-Embedded (FFPE) Duodenal Biopsies
Erratum in
-
Correction to: A New Intraepithelial γδ T-Lymphocyte Marker for Celiac Disease Classification in Formalin-Fixed Paraffin-Embedded (FFPE) Duodenal Biopsies.Dig Dis Sci. 2021 Dec;66(12):4572. doi: 10.1007/s10620-020-06731-3. Dig Dis Sci. 2021. PMID: 33236317 Free PMC article. No abstract available.
Abstract
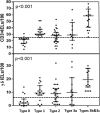
Background: The histopathologic diagnosis of celiac disease (CD) may be challenging when the duodenal biopsies mucosal injury is limited. Intraepithelial T-lymphocytes (IELs) can be useful to characterize the degree of mucosal inflammation. A small fraction of IELs expresses the γδ T-cell receptor (named γδ-IELs), whose density, determined by flow cytometry or frozen section immunohistochemistry (IHC), is a specific marker for CD.
Aim: To establish a new IHC assay for γδ-IELs applicable to formalin-fixed paraffin-embedded (FFPE) duodenal biopsies.
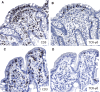
Methods: We analyzed γδ-IELs using IHC in 138 duodenal biopsies using a standard IHC staining protocol with a new monoclonal antibody H-41. IELs were quantitated with digital image analysis.
Results: Compared to those in non-celiac controls (n = 51), γδ-IEL density was significantly increased in newly diagnosed celiac disease patients (n = 22, p < 0.0001). In ROC-curve analysis, the cutoff of 6.5 γδ-IELs/100 enterocytes distinguished optimally active CD patients from non-celiac controls (sensitivity 96%, specificity 95%). γδ-IEL density in CD patients on a gluten-free diet (n = 53) were also higher than in controls (p < 0.0001), but lower than those in newly diagnosed CD (p < 0.0001). The diagnostic value of γδ-IELs outperformed that of CD3 + IELs in both patient groups. γδ-IELs were better than CD3 + IELs distinguishing between celiac disease and conditions histologically mimicking celiac disease (n = 12).
Conclusions: Intraepithelial γδ T-lymphocytes can be stained and quantitated reliably in FFPE duodenal biopsies. The results showed excellent specificity and sensitivity for celiac disease. The new IHC method of detection of γδ-IELs is a promising addition to the routine histopathologic assessment methodology of celiac disease.
Keywords: Autoimmune; Celiac disease; Diagnostics; Histopathology; Inflammation; Lymphocyte; Small bowel.
© 2020. The Author(s).
Conflict of interest statement
None declared.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

