High tumor mutational burden and T-cell activation are associated with long-term response to anti-PD1 therapy in Lynch syndrome recurrent glioblastoma patient
- PMID: 33140187
- PMCID: PMC10992921
- DOI: 10.1007/s00262-020-02769-4
High tumor mutational burden and T-cell activation are associated with long-term response to anti-PD1 therapy in Lynch syndrome recurrent glioblastoma patient
Abstract
Background: Glioblastomas (GBMs) in patients harboring somatic or germinal mutations of mismatch-repair (MMR) genes exhibit a hypermutable phenotype. Here, we describe a GBM patient with increased tumor mutational burden and germline MMR mutations, treated using anti-PD1 therapy.
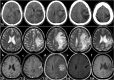
Methods: A woman with newly diagnosed GBM (nGBM) was treated by surgery, radiotherapy, and temozolomide. The tumor recurred after 13 months leading to a second surgery and treatment with nivolumab. Whole-exome sequencing was performed on the nGBM, recurrent GBM (rGBM), and blood. Immune infiltration was investigated by immunohistochemistry and the immune response in the blood during treatment was analyzed by flow cytometry.
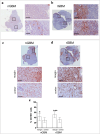
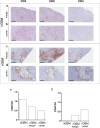
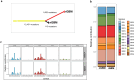
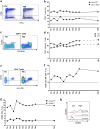
Results: High density of infiltrating CD163 + cells was found in both GBM specimens. Large numbers of CD3 + and CD8 + T cells were homogeneously distributed in the nGBM. The infiltration of CD4 + T cells and a different CD8 + T cell density were observed in the rGBM. Both GBM shared 12,431 somatic mutations, with 113 substitutions specific to the nGBM and 1,683 specific to the rGBM. Germline variants included pathogenic mutation in the MSH2 (R359S) gene, suggesting the diagnosis of Lynch syndrome. Systemic immunophenotyping revealed the generation of CD8 + T memory cells and persistent activation of CD4 + T cells. The patient is still receiving nivolumab 68 months after the second surgery.
Conclusions: Our observations indicate that the hypermutator phenotype associated with germinal mutations of MMR genes and abundant T-cell infiltration contributes to a durable clinical benefit sustained by a persistent and robust immune response during anti-PD1 therapy.
Keywords: Glioblastoma (GBM); Lynch syndrome (LS); Mismatch repair (MMR); Nivolumab/anti-PD1 therapy; Tumor infiltration lymphocytes (TILs); Tumor mutational burden (TMB).
Conflict of interest statement
There are no competing interests in the report.
Figures
Similar articles
-
A Durable Response to Pembrolizumab in a Patient with Uterine Serous Carcinoma and Lynch Syndrome due to the MSH6 Germline Mutation.Oncologist. 2021 Oct;26(10):811-817. doi: 10.1002/onco.13832. Epub 2021 Jun 14. Oncologist. 2021. PMID: 34018286 Free PMC article.
-
Immunogenomics of Colorectal Cancer Response to Checkpoint Blockade: Analysis of the KEYNOTE 177 Trial and Validation Cohorts.Gastroenterology. 2021 Oct;161(4):1179-1193. doi: 10.1053/j.gastro.2021.06.064. Epub 2021 Jun 29. Gastroenterology. 2021. PMID: 34197832 Free PMC article.
-
Genetic, Epigenetic, and Immunologic Profiling of MMR-Deficient Relapsed Glioblastoma.Clin Cancer Res. 2019 Mar 15;25(6):1828-1837. doi: 10.1158/1078-0432.CCR-18-1892. Epub 2018 Dec 4. Clin Cancer Res. 2019. PMID: 30514778
-
Lynch syndrome and Lynch syndrome mimics: The growing complex landscape of hereditary colon cancer.World J Gastroenterol. 2015 Aug 21;21(31):9253-61. doi: 10.3748/wjg.v21.i31.9253. World J Gastroenterol. 2015. PMID: 26309352 Free PMC article. Review.
-
Complete Response to Immune Checkpoint Inhibition in a Platinum Resistant Primary Ovarian Cancer Patient With Lynch Syndrome: A Case Report and Review of the Literature.Anticancer Res. 2023 Apr;43(4):1655-1662. doi: 10.21873/anticanres.16317. Anticancer Res. 2023. PMID: 36974818 Review.
Cited by
-
Integrated machine learning methods identify FNDC3B as a potential prognostic biomarker and correlated with immune infiltrates in glioma.Front Immunol. 2022 Oct 6;13:1027154. doi: 10.3389/fimmu.2022.1027154. eCollection 2022. Front Immunol. 2022. PMID: 36275754 Free PMC article.
-
Systematic Review of Molecular Targeted Therapies for Adult-Type Diffuse Glioma: An Analysis of Clinical and Laboratory Studies.Int J Mol Sci. 2023 Jun 21;24(13):10456. doi: 10.3390/ijms241310456. Int J Mol Sci. 2023. PMID: 37445633 Free PMC article.
-
Durable benefit and change in TCR clonality with nivolumab in a Lynch syndrome-associated glioma.Ther Adv Med Oncol. 2022 Jun 8;14:17588359221100863. doi: 10.1177/17588359221100863. eCollection 2022. Ther Adv Med Oncol. 2022. PMID: 35694191 Free PMC article.
-
Deciphering the Labyrinthine System of the Immune Microenvironment in Recurrent Glioblastoma: Recent Original Advances and Lessons from Clinical Immunotherapeutic Approaches.Cancers (Basel). 2021 Dec 7;13(24):6156. doi: 10.3390/cancers13246156. Cancers (Basel). 2021. PMID: 34944776 Free PMC article. Review.
-
Development of a T-cell activation-related module with predictive value for the prognosis and immune checkpoint blockade therapy response in glioblastoma.PeerJ. 2021 Dec 22;9:e12547. doi: 10.7717/peerj.12547. eCollection 2021. PeerJ. 2021. PMID: 35036121 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

