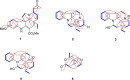
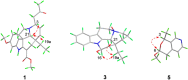
Bousmekines A-E, New Alkaloids from Two Bousigonia Species: B.angustifolia and B. mekongensis
- PMID: 33140311
- PMCID: PMC7981358
- DOI: 10.1007/s13659-020-00278-6
Bousmekines A-E, New Alkaloids from Two Bousigonia Species: B.angustifolia and B. mekongensis
Abstract
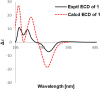
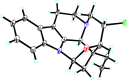
Four new monoterpenoid indole alkaloids, bousmekines A-D (1-4), and one new pyranopyridine alkaloid, bousmekine E (5), were isolated from the twigs and leaves of Bousigonia angustifolia and Bousigonia mekongensis. Their structures including absolute configurations were elucidated by a combination of MS, NMR, ECD calculation, and single-crystal X-ray diffraction analysis. Compound 2 was an eburnea-type MIAs characterized by a rare chlorine atom while 5 possessed a novel pyranopyridine moiety. Their cytotoxicities against several human cancer cell lines were evaluated and compound 1 exhibited significant cytotoxicity with IC50 values of 0.8-7.4 μM.
Keywords: B. angustifolia; B. mekongensis; Bousigonia; Bousmekines A-E; Monoterpenoid indole alkaloids.
Conflict of interest statement
Authors declare that there is no conflict of interest.
Figures
References
-
- P. M. Dewick, Alkaloids. In Medicinal Natural Product: A Biosynthetic Approach; John Wiley & Sons Ltd: Chichester, (2002) 340–341.
Grants and funding
LinkOut - more resources
Full Text Sources