The Human Explanted Heart Program: A translational bridge for cardiovascular medicine
- PMID: 33141063
- PMCID: PMC7581399
- DOI: 10.1016/j.bbadis.2020.165995
The Human Explanted Heart Program: A translational bridge for cardiovascular medicine
Abstract
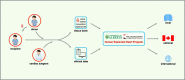
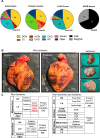
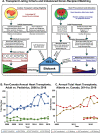
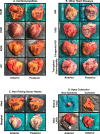
The progression of cardiovascular research is often impeded by the lack of reliable disease models that fully recapitulate the pathogenesis in humans. These limitations apply to both in vitro models such as cell-based cultures and in vivo animal models which invariably are limited to simulate the complexity of cardiovascular disease in humans. Implementing human heart tissue in cardiovascular research complements our research strategy using preclinical models. We established the Human Explanted Heart Program (HELP) which integrates clinical, tissue and molecular phenotyping thereby providing a comprehensive evaluation into human heart disease. Our collection and storage of biospecimens allow them to retain key pathogenic findings while providing novel insights into human heart failure. The use of human non-failing control explanted hearts provides a valuable comparison group for the diseased explanted hearts. Using HELP we have been able to create a tissue repository which have been used for genetic, molecular, cellular, and histological studies. This review describes the process of collection and use of explanted human heart specimens encompassing a spectrum of pediatric and adult heart diseases, while highlighting the role of these invaluable specimens in translational research. Furthermore, we highlight the efficient procurement and bio-preservation approaches ensuring analytical quality of heart specimens acquired in the context of heart donation and transplantation.
Keywords: Heart failure; Human explanted heart; Pediatric heart diseases; Tissue biobank; Translational research; Ventricular assist device.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Benjamin E.J. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–e528. - PubMed
-
- Braunwald E., Bristow M.R. Congestive heart failure: fifty years of progress. Circulation. 2000;102(20 Suppl 4):Iv14–23. - PubMed
-
- Hill J.A., Olson E.N. Cardiac plasticity. N. Engl. J. Med. 2008;358(13):1370–1380. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

