Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome
- PMID: 33141117
- PMCID: PMC7685724
- DOI: 10.1172/JCI140617
Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome
Abstract
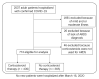
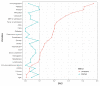
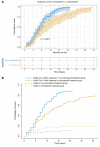
BACKGROUNDCorticosteroids are widely used in patients with COVID 19, although their benefit-to-risk ratio remains controversial.METHODSPatients with severe COVID-19-related acute respiratory distress syndrome (ARDS) were included from December 29, 2019 to March 16, 2020 in 5 tertiary Chinese hospitals. Cox proportional hazards and competing risks analyses were conducted to analyze the impact of corticosteroids on mortality and SARS-CoV-2 RNA clearance, respectively. We performed a propensity score (PS) matching analysis to control confounding factors.RESULTSOf 774 eligible patients, 409 patients received corticosteroids, with a median time from hospitalization to starting corticosteroids of 1.0 day (IQR 0.0-3.0 days) . As compared with usual care, treatment with corticosteroids was associated with increased rate of myocardial (15.6% vs. 10.4%, P = 0.041) and liver injury (18.3% vs. 9.9%, P = 0.001), of shock (22.0% vs. 12.6%, P < 0.001), of need for mechanical ventilation (38.1% vs. 19.5%, P < 0.001), and increased rate of 28-day all-cause mortality (44.3% vs. 31.0%, P < 0.001). After PS matching, corticosteroid therapy was associated with 28-day mortality (adjusted HR 1.46, 95% CI 1.01-2.13, P = 0.045). High dose (>200 mg) and early initiation (≤3 days from hospitalization) of corticosteroid therapy were associated with a higher 28-day mortality rate. Corticosteroid use was also associated with a delay in SARS-CoV-2 coronavirus RNA clearance in the competing risk analysis (subhazard ratio 1.59, 95% CI 1.17-2.15, P = 0.003).CONCLUSIONAdministration of corticosteroids in severe COVID-19-related ARDS is associated with increased 28-day mortality and delayed SARS-CoV-2 coronavirus RNA clearance after adjustment for time-varying confounders.FUNDINGNone.
Keywords: COVID-19; Respiration.
Conflict of interest statement
Figures


Comment in
-
Corticosteroids, COVID-19 pneumonia, and acute respiratory distress syndrome.J Clin Invest. 2020 Dec 1;130(12):6218-6221. doi: 10.1172/JCI143331. J Clin Invest. 2020. PMID: 32976118 Free PMC article.
References
-
- World Health Organization. Coronavirus disease (COVID-19) weekly epidemiological update and weekly operational update. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2... Accessed July 17, 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

