European Stakeholder Learnings Regarding Biosimilars: Part I-Improving Biosimilar Understanding and Adoption
- PMID: 33141421
- PMCID: PMC7669769
- DOI: 10.1007/s40259-020-00452-9
European Stakeholder Learnings Regarding Biosimilars: Part I-Improving Biosimilar Understanding and Adoption
Abstract
Background: Despite the benefits offered by biosimilars in terms of cost savings and improved patient access to biological therapies, and an established regulatory pathway in Europe, biosimilar adoption is challenged by a lack of knowledge and understanding among stakeholders such as healthcare professionals and patients about biosimilars, impacting their trust and willingness to use them. In addition, stakeholders are faced with questions about clinical implementation aspects such as switching.
Objective: This study aims to provide recommendations on how to improve biosimilar understanding and adoption among stakeholders based on insights of healthcare professionals (physicians, hospital pharmacists, nurses), patient(s) (representatives) and regulators across Europe.
Method: The study consists of a structured literature review gathering original research data on stakeholder knowledge about biosimilars, followed by semi-structured interviews across five stakeholder groups including physicians, hospital pharmacists, nurses, patient(s) (representatives) and regulators across Europe.
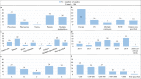
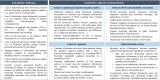
Results: Although improvement in knowledge was observed over time, generally low to moderate levels of awareness, knowledge and trust towards biosimilars among healthcare professionals and patients are identified in literature (N studies = 106). Based on the provided insights from interviews with European experts (N = 44), a number of challenges regarding biosimilar stakeholder understanding are identified, including a lack of practical information about biosimilars and their use, a lack of understanding about biosimilar concepts and a lack of knowledge about biologicals in general. Misinformation by originator industry is also believed to have impacted stakeholder trust. In terms of possible solutions and actions to improve stakeholder understanding, broad support exists to (1) organize initiatives focussed on explaining the rationale behind biosimilar concepts and the approval pathway, (2) invest in education about biologicals in general, (3) develop clear and one-voice regulatory guidance about biosimilar interchangeability and switching across Europe, (4) disseminate real-world clinical biosimilar (switch) data, (5) share biosimilar experiences by key opinion leaders and among peers, (6) provide practical biosimilar product information, (7) provide guidance about biosimilar use, (8) actively counterbalance misinformation and organize information initiatives by neutral entities, (9) organize multi-stakeholder informational and educational efforts, aligning information between involved stakeholder groups and (10) design initiatives in a way that ensures active information uptake. Furthermore, interviewees argue that governments should be proactive in these regards.
Conclusions: This study argues in favour of a structural, multi-stakeholder framework at both European and national level to improve stakeholder biosimilar understanding and acceptance. It proposes a number of actionable recommendations that can inform policy making and guide stakeholders, which can contribute to realizing healthcare system benefits offered by biosimilar competition.
Conflict of interest statement
IH, SS and AGV are founders of the KU Leuven Fund on Market Analysis of Biologics and Biosimilars following Loss of Exclusivity (MABEL Fund). AGV is involved in consulting, advisory work and speaking engagements for a number of companies, i.e., AbbVie, Accord, Amgen, Biogen, EGA, Pfizer/Hospira, Mundipharma, Roche, Novartis, Sandoz and Boehringer Ingelheim. SS was involved in a stakeholder roundtable on biologics and biosimilars sponsored by Amgen, Pfizer and MSD. He has participated in advisory board meetings for Pfizer and Amgen; and he has contributed to studies on biologics and biosimilars for Hospira, Celltrion, Mundipharma and Pfizer. IH and LB declare no conflict of interest. LB declares that the research was conducted in the absence of any commercial or financial relationship that could be perceived as a potential conflict of interest.
Figures
Similar articles
-
European Stakeholder Learnings Regarding Biosimilars: Part II-Improving Biosimilar Use in Clinical Practice.BioDrugs. 2020 Dec;34(6):797-808. doi: 10.1007/s40259-020-00440-z. BioDrugs. 2020. PMID: 33063267 Free PMC article.
-
Regulatory Information and Guidance on Biosimilars and Their Use Across Europe: A Call for Strengthened One Voice Messaging.Front Med (Lausanne). 2022 Mar 9;9:820755. doi: 10.3389/fmed.2022.820755. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35355594 Free PMC article.
-
Biosimilar Use and Switching in Belgium: Avenues for Integrated Policymaking.Front Pharmacol. 2022 Jul 12;13:821616. doi: 10.3389/fphar.2022.821616. eCollection 2022. Front Pharmacol. 2022. PMID: 35903323 Free PMC article.
-
Biosimilars in Belgium: a proposal for a more competitive market.Acta Clin Belg. 2021 Dec;76(6):441-452. doi: 10.1080/17843286.2020.1761690. Epub 2020 May 13. Acta Clin Belg. 2021. PMID: 32400319 Review.
-
Informing Patients about Biosimilar Medicines: The Role of European Patient Associations.Pharmaceuticals (Basel). 2021 Feb 4;14(2):117. doi: 10.3390/ph14020117. Pharmaceuticals (Basel). 2021. PMID: 33557030 Free PMC article. Review.
Cited by
-
Can Endangered Biosimilar Markets be Rescued? The Need to Bridge Competing Interests for Long-Term Gain.BioDrugs. 2024 May;38(3):325-329. doi: 10.1007/s40259-024-00652-7. Epub 2024 Feb 26. BioDrugs. 2024. PMID: 38407791
-
Budget Impact Analysis of the Introduction of a Trastuzumab Biosimilar for HER2-Positive Breast Cancer in China.Clin Drug Investig. 2022 Nov;42(11):937-947. doi: 10.1007/s40261-022-01197-9. Epub 2022 Sep 17. Clin Drug Investig. 2022. PMID: 36115003
-
Biosimilars in the Era of Artificial Intelligence-International Regulations and the Use in Oncological Treatments.Pharmaceuticals (Basel). 2024 Jul 10;17(7):925. doi: 10.3390/ph17070925. Pharmaceuticals (Basel). 2024. PMID: 39065775 Free PMC article. Review.
-
Impact of Value-Driven Healthcare Strategies for Biosimilar Adoption: The Singapore Story.Pharmacoecon Open. 2024 Sep;8(5):679-688. doi: 10.1007/s41669-024-00491-w. Epub 2024 Jul 23. Pharmacoecon Open. 2024. PMID: 39042227 Free PMC article.
-
Biosimilar perceptions among autoimmune prescribers and pharmacists in health system specialty pharmacy.J Manag Care Spec Pharm. 2024 Feb 3;30(2):175-182. doi: 10.18553/jmcp.2024.30.2.175. J Manag Care Spec Pharm. 2024. PMID: 38308629 Free PMC article.
References
-
- European Medicines Agency. Guideline on similar biological medicinal products. 2014. https://www.ema.europa.eu/en/similar-biological-medicinal-products.
-
- European Medicines Agency and the European Commission. Biosimilars in the EU - Information guide for healthcare professionals. 2017. https://www.ema.europa.eu/documents/leaflet/biosimilars-eu-information-g....
-
- IQVIA. The Impact of Biosimilar Competition in Europe. 2018. https://ec.europa.eu/docsroom/documents/31642/attachments/1/translations....
-
- IQVIA. Advancing Biosimilar Sustainability in Europe—a Multi-Stakeholder Assessment. 2018https://www.iqvia.com/insights/the-iqvia-institute/reports/advancing-bio....
-
- European Medicines Agency. Biosimilar medicines. https://www.ema.europa.eu/en/medicines/search_api_aggregation_ema_medici.... Accessed 3 Jan 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

