A Mouse Model of Sublethal Leptospirosis: Protocols for Infection with Leptospira Through Natural Transmission Routes, for Monitoring Clinical and Molecular Scores of Disease, and for Evaluation of the Host Immune Response
- PMID: 33141517
- PMCID: PMC7643393
- DOI: 10.1002/cpmc.127
A Mouse Model of Sublethal Leptospirosis: Protocols for Infection with Leptospira Through Natural Transmission Routes, for Monitoring Clinical and Molecular Scores of Disease, and for Evaluation of the Host Immune Response
Abstract
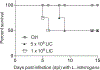
Leptospirosis is a zoonotic disease caused by pathogenic Leptospira species that are maintained in sylvatic and domestic environments by transmission among rodents and other carriers. Humans become infected after contact of breached skin or mucosa with contaminated water or soil. Understanding persistent or sublethal infection in a host is critical for controlling human risk of exposure to pathogenic Leptospira. Animal models that recapitulate disease progression after infection via natural transmission routes are more appropriate for validation of vaccines and therapeutics. Furthermore, the ability to measure shedding of live Leptospira in urine of reservoir and carrier hosts can be used to develop new diagnostic assays and sensors to evaluate human risk of exposure. We developed inbred mouse models of Leptospirosis, that bypass survival as a criterion, in which we can analyze both pathogen and host factors affecting sublethal infection (<1 month), including shedding of Leptospira in urine. Mice are infected with pathogenic Leptospira using a physiologic route, and the clinical, histological, and molecular scores of disease are measured. Furthermore, the host immune response to Leptospira is evaluated. This mouse model also provides a tool in which to test fundamental hypotheses related to host-pathogen interactions and the immune mechanisms engaged in protective and pathogenic immune responses. © 2020 Wiley Periodicals LLC Basic Protocol 1: Culture and maintenance of virulent Leptospira Basic Protocol 2: Infection of mice through a physiologic route and collection of clinical scores and biological samples Basic Protocol 3: Analysis of pathogenesis after Leptospira infection.
Keywords: Leptospira; mouse model; natural transmission routes; physiologic route of infection; sublethal leptospirosis.
© 2020 Wiley Periodicals LLC.
Figures


Similar articles
-
Review paper: Host-pathogen interactions in the kidney during chronic leptospirosis.Vet Pathol. 2009 Sep;46(5):792-9. doi: 10.1354/vp.08-VP-0265-N-REV. Epub 2009 May 9. Vet Pathol. 2009. PMID: 19429975 Review.
-
Inbred Rats as a Model to Study Persistent Renal Leptospirosis and Associated Cellular Immune Responsiveness.Front Cell Infect Microbiol. 2018 Mar 14;8:66. doi: 10.3389/fcimb.2018.00066. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29594063 Free PMC article.
-
Proteomic profiles of Leptospira borgpetersenii serovar Hardjo strains JB197 and HB203 cultured at different temperatures.J Proteomics. 2024 Mar 20;295:105106. doi: 10.1016/j.jprot.2024.105106. Epub 2024 Feb 5. J Proteomics. 2024. PMID: 38320623
-
A live attenuated-vaccine model confers cross-protective immunity against different species of the Leptospira genus.Elife. 2021 Jan 26;10:e64166. doi: 10.7554/eLife.64166. Elife. 2021. PMID: 33496263 Free PMC article.
-
Leptospira and leptospirosis.Vet Microbiol. 2010 Jan 27;140(3-4):287-96. doi: 10.1016/j.vetmic.2009.03.012. Epub 2009 Mar 13. Vet Microbiol. 2010. PMID: 19345023 Review.
Cited by
-
Enzyme immunoassays (EIA) for serodiagnosis of human leptospirosis: specific IgG3/IgG1 isotyping may further inform diagnosis of acute disease.PLoS Negl Trop Dis. 2022 Feb 23;16(2):e0010241. doi: 10.1371/journal.pntd.0010241. eCollection 2022 Feb. PLoS Negl Trop Dis. 2022. PMID: 35196321 Free PMC article.
-
Hematogenous dissemination of pathogenic and non-pathogenic Leptospira in a short-term murine model of infection.Front Cell Infect Microbiol. 2022 Jul 18;12:917962. doi: 10.3389/fcimb.2022.917962. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35923802 Free PMC article.
-
Role of TLR4 in Persistent Leptospira interrogans Infection: A Comparative In Vivo Study in Mice.Front Immunol. 2021 Jan 15;11:572999. doi: 10.3389/fimmu.2020.572999. eCollection 2020. Front Immunol. 2021. PMID: 33519799 Free PMC article.
-
TLR4 competence and mouse models of leptospirosis.bioRxiv [Preprint]. 2025 Feb 4:2025.02.03.636333. doi: 10.1101/2025.02.03.636333. bioRxiv. 2025. Update in: PLoS Negl Trop Dis. 2025 May 30;19(5):e0013163. doi: 10.1371/journal.pntd.0013163. PMID: 39975062 Free PMC article. Updated. Preprint.
-
Transient Presence of Live Leptospira interrogans in Murine Testes.Microbiol Spectr. 2022 Jun 29;10(3):e0277521. doi: 10.1128/spectrum.02775-21. Epub 2022 Apr 21. Microbiol Spectr. 2022. PMID: 35446113 Free PMC article.
References
-
- Asoh T, Saito M, Villanueva SY, Kanemaru T, Gloriani N and Yoshida S (2014). “Natural defense by saliva and mucosa against oral infection by Leptospira.” Can J Microbiol 60(6): 383–389. - PubMed
-
- Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, Vinetz JM and C. Peru-United States Leptospirosis (2003). “Leptospirosis: a zoonotic disease of global importance.” Lancet Infect Dis 3(12): 757–771. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

