The matrix metalloproteinase inhibitor IPR-179 has antiseizure and antiepileptogenic effects
- PMID: 33141761
- PMCID: PMC7773344
- DOI: 10.1172/JCI138332
The matrix metalloproteinase inhibitor IPR-179 has antiseizure and antiepileptogenic effects
Abstract
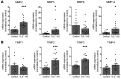
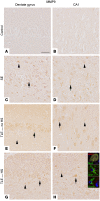
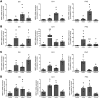
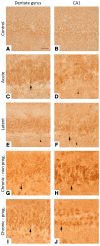
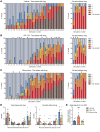
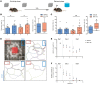
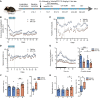
Matrix metalloproteinases (MMPs) are synthesized by neurons and glia and released into the extracellular space, where they act as modulators of neuroplasticity and neuroinflammatory agents. Development of epilepsy (epileptogenesis) is associated with increased expression of MMPs, and therefore, they may represent potential therapeutic drug targets. Using quantitative PCR (qPCR) and immunohistochemistry, we studied the expression of MMPs and their endogenous inhibitors tissue inhibitors of metalloproteinases (TIMPs) in patients with status epilepticus (SE) or temporal lobe epilepsy (TLE) and in a rat TLE model. Furthermore, we tested the MMP2/9 inhibitor IPR-179 in the rapid-kindling rat model and in the intrahippocampal kainic acid mouse model. In both human and experimental epilepsy, MMP and TIMP expression were persistently dysregulated in the hippocampus compared with in controls. IPR-179 treatment reduced seizure severity in the rapid-kindling model and reduced the number of spontaneous seizures in the kainic acid model (during and up to 7 weeks after delivery) without side effects while improving cognitive behavior. Moreover, our data suggest that IPR-179 prevented an MMP2/9-dependent switch-off normally restraining network excitability during the activity period. Since increased MMP expression is a prominent hallmark of the human epileptogenic brain and the MMP inhibitor IPR-179 exhibits antiseizure and antiepileptogenic effects in rodent epilepsy models and attenuates seizure-induced cognitive decline, it deserves further investigation in clinical trials.
Keywords: Epilepsy; Extracellular matrix; Neuroscience; Seizures; Therapeutics.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

