Long-term survival of patients with relapsed/refractory acute lymphoblastic leukemia treated with blinatumomab
- PMID: 33141929
- PMCID: PMC7894150
- DOI: 10.1002/cncr.33298
Long-term survival of patients with relapsed/refractory acute lymphoblastic leukemia treated with blinatumomab
Abstract
Background: Blinatumomab is a CD19 BiTE (bispecific T-cell engager) immuno-oncology therapy that mediates the lysis of cells expressing CD19.
Methods: A pooled analysis of long-term follow-up data from 2 phase 2 studies that evaluated blinatumomab in heavily pretreated adults with Philadelphia chromosome-negative, relapsed/refractory B-cell precursor acute lymphoblastic leukemia was conducted.
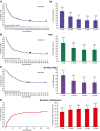
Results: A total of 259 patients were included in the analysis. The median overall survival (OS) among all patients, regardless of response, was 7.5 months (95% confidence interval [CI], 5.5-8.5 months); the median follow-up time for OS was 36.0 months (range, 0.3-60.8 months). The median relapse-free survival (RFS) among patients who achieved a complete remission (CR) or complete remission with partial hematologic recovery (CRh) in the first 2 cycles (n = 123) was 7.7 months (95% CI, 6.2-10.0 months); the median follow-up time for RFS was 35.0 months (range, 9.5-59.5 months). OS and RFS plateaued with 3-year rates of 17.7% and 23.4%, respectively. The cumulative incidence function of the time to relapse, with death not due to relapse considered a competing risk, for patients who achieved a CR/CRh within 2 cycles of treatment also plateaued with a 3-year relapse rate of 59.3%. For patients who achieved a CR/CRh with blinatumomab followed by allogeneic hematopoietic stem cell transplantation while in continuous CR, the median OS was 18.1 months (95% CI, 10.3-30.0 months) with a 3-year survival rate of 37.2%.
Conclusions: These data suggest that long-term survival is possible after blinatumomab therapy.
Lay summary: Immuno-oncology therapies such as blinatumomab activate the patient's own immune system to kill cancer cells. This study combined follow-up data from 2 blinatumomab-related clinical trials to evaluate long-term survival in patients with relapsed and/or refractory B-cell precursor acute lymphoblastic leukemia at high risk for unfavorable outcomes. Among patients who achieved a deep response with blinatumomab, one-third lived 3 years or longer. These findings suggest that long-term survival is possible after treatment with blinatumomab.
Keywords: acute lymphoblastic leukemia (ALL); bispecific T-cell engager (BiTE); blinatumomab; overall survival.
© 2020 Amgen GmbH. Cancer published by Wiley Periodicals LLC on behalf of American Cancer Society.
Conflict of interest statement
Max S. Topp reports a research grant and personal fees from Amgen. Nicola Gökbuget reports research funding, personal fees, and travel support from and participation in advisory boards for Amgen, Novartis, Pfizer, and Celgene. Gerhard Zugmaier is an employee and stockholder of Amgen and reports patent royalties from Amgen. Anthony S. Stein reports participation in speakers' bureaus for Amgen and Celgene and personal fees from Amgen. Hervé Dombret is an advisor for, serves on a speakers' bureau for, and reports research support, consultancy, honoraria, and travel/accommodation support from Amgen; is an advisor for and reports research support and honoraria from Roche/Genentech; is an advisor for, serves on a speakers' bureau for, and reports honoraria and travel/accommodation support from Pfizer; is an advisor for, serves on a speakers' bureau for, and reports research support, honoraria, and travel/accommodation support from Ariad (Incyte); is an advisor for and reports research support and honoraria from Jazz Pharma and Kite Pharma; is an advisor for and reports honoraria from Novartis, Agios, Sunesis, Ambit (Daiichi Sankyo), Karyopharm, Menarini, Astellas, Janssen, Servier, Seattle Genetics, and Cellectis; and is a consultant and advisor for, serves on a speakers' bureau for, and reports honoraria from Celgene. Yuqi Chen is an employee and stockholder of Amgen. Josep‐Maria Ribera reports research grants from Amgen, Novartis, and Pfizer and personal fees from Amgen, Celgene, Jazz Pharmaceuticals, Novartis, Pfizer, Shire, and Takeda. Ralf C. Bargou reports consultancy honoraria from AstraZeneca, Genmab, Pfizer, Amgen, Cellex, GEMoaB Monoclonals, Molecular Partners, and Novartis and royalty payments for a blinatumomab patent. Heinz‐August Horst reports research funding and travel support from and participation in advisory boards for Amgen; participation in advisory boards for Pfizer, Jazz Pharmaceuticals, and Novartis; and research funding from Regeneron. Hagop M. Kantarjian reports research funding from AbbVie, Agios Pharmaceuticals, Amgen, Ariad Pharmaceuticals, Astex Pharmaceuticals, Bristol‐Myers Squibb, Cyclacel Pharmaceuticals, Daiichi‐Sankyo, ImmunoGen, Jazz Pharmaceuticals, Novartis, and Pfizer and honoraria from AbbVie, Actinium Pharmaceuticals, Agios Pharmaceuticals, Amgen, ImmunoGen, Orsenix, Pfizer, and Takeda.
Figures
References
-
- Gökbuget N, Stanze D, Beck J, et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood. 2012;120:2032‐2041. - PubMed
-
- Tavernier E, Boiron JM, Huguet F, et al. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA‐94 trial. Leukemia. 2007;21:1907‐1914. - PubMed
-
- Jabbour EJ, Gokbuget N, Kantarjian HM, et al. Transplantation in adults with relapsed/refractory acute lymphoblastic leukemia who are treated with blinatumomab from a phase 3 study. Cancer. 2019;125:4181‐4192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

