A Rapid Immunochromatographic Method Based on a Secondary Antibody-Labelled Magnetic Nanoprobe for the Detection of Hepatitis B preS2 Surface Antigen
- PMID: 33142715
- PMCID: PMC7692799
- DOI: 10.3390/bios10110161
A Rapid Immunochromatographic Method Based on a Secondary Antibody-Labelled Magnetic Nanoprobe for the Detection of Hepatitis B preS2 Surface Antigen
Abstract
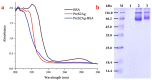
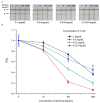
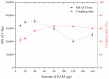
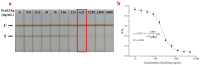
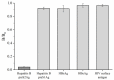
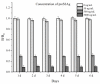
Hepatitis B is a globally prevalent viral infectious disease caused by the hepatitis B virus (HBV). In this study, an immunochromatographic assay (ICA) for the rapid detection of hepatitis B preS2 antigen (preS2Ag) was established. The magnetic nanoparticles (MNPs) indirectly labelled with goat anti-mouse (GAM) secondary antibody were applied as a nanoprobe for free preS2 antibody (preS2Ab) capturing and signal amplification. By employing sample pre-incubation processing as well, preS2Ag-preS2Ab was sufficiently caught by the GAM-MNPs probe in 5 min. A qualitative sensitivity of 625 ng/mL was obtained by naked-eye observation within 15-20 min. A standard curve (0-5000 ng/mL) was established, with a quantitative limit of detection (LOD) of 3.6 ng/mL, based on the stability and penetrability of the magnetic signal characteristics. The proposed method for preS2Ag was rapid (~25 min, cf. ELISA ~4 h) and had a good accuracy, which was verified using an ELISA kit (relative error < 15%). Large equipment and skilled technicians were not required. The sensitivity and specificity of the developed GAM-MNPs-ICA method were 93.3% and 90% in clinical serum samples (n = 25), respectively. A good detection consistency (84%) was observed between the developed ICA method and 2 types of commercial ELISA kits, indicating that the GAM-MNPs-ICA has a potential application in large-scale screening for and point-of-care diagnosis of hepatitis B or other infectious diseases.
Keywords: hepatitis B; hepatitis B preS2 antigen; immunochromatographic assay; magnetic nanoparticles; rapid detection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
PreS antigen expression and anti-preS response in hepatitis B virus infections: relationship to serum HBV-DNA, intrahepatic HBcAg, liver damage and specific T-cell response.Arch Virol Suppl. 1992;4:105-12. doi: 10.1007/978-3-7091-5633-9_22. Arch Virol Suppl. 1992. PMID: 1280500
-
Adenovirus-expressed preS2 antibody inhibits hepatitis B virus infection and hepatic carcinogenesis.World J Gastroenterol. 2012 Jan 28;18(4):349-55. doi: 10.3748/wjg.v18.i4.349. World J Gastroenterol. 2012. PMID: 22294841 Free PMC article.
-
Immunochromatographic assay for quantitative and sensitive detection of hepatitis B virus surface antigen using highly luminescent quantum dot-beads.Talanta. 2015 Sep 1;142:145-9. doi: 10.1016/j.talanta.2015.04.058. Epub 2015 Apr 27. Talanta. 2015. PMID: 26003704
-
Quantitative assessment of IgM antibodies towards an immunodominant B-cell epitope within the preS2 domain of HBV in the natural course and during combined prednisone/interferon alpha 2b treatment of chronic hepatitis B virus infection.J Med Virol. 1995 Jun;46(2):138-43. doi: 10.1002/jmv.1890460210. J Med Virol. 1995. PMID: 7636501
-
Molecular and Serological Characterization of Hepatitis B Virus (HBV)-Positive Samples with Very Low or Undetectable Levels of HBV Surface Antigen.Viruses. 2021 Oct 13;13(10):2053. doi: 10.3390/v13102053. Viruses. 2021. PMID: 34696483 Free PMC article.
Cited by
-
Lateral Flow Assay for Hepatitis B Detection: A Review of Current and New Assays.Micromachines (Basel). 2023 Jun 12;14(6):1239. doi: 10.3390/mi14061239. Micromachines (Basel). 2023. PMID: 37374824 Free PMC article. Review.
-
Colorimetric and Raman dual-mode lateral flow immunoassay detection of SARS-CoV-2 N protein antibody based on Ag nanoparticles with ultrathin Au shell assembled onto Fe3O4 nanoparticles.Anal Bioanal Chem. 2023 Feb;415(4):545-554. doi: 10.1007/s00216-022-04437-1. Epub 2022 Nov 22. Anal Bioanal Chem. 2023. PMID: 36414739 Free PMC article.
-
A Rapid Tricolour Immunochromatographic Assay for Simultaneous Detection of Tricaine and Malachite Green.Biosensors (Basel). 2022 Jun 26;12(7):456. doi: 10.3390/bios12070456. Biosensors (Basel). 2022. PMID: 35884259 Free PMC article.
References
-
- Yen T.T.C., Yang A., Chiu W.T., Li T.N., Wang L.H., Wu Y.H., Wang H.C., Chen L., Wang W.C., Huang W., et al. Hepatitis B virus PreS2-mutant large surface antigen activates store-operated calcium entry and promotes chromosome instability. Oncotarget. 2016;7:23346–23360. doi: 10.18632/oncotarget.8109. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

