Taurine Enhances Iron-Related Proteins and Reduces Lipid Peroxidation in Differentiated C2C12 Myotubes
- PMID: 33142756
- PMCID: PMC7693586
- DOI: 10.3390/antiox9111071
Taurine Enhances Iron-Related Proteins and Reduces Lipid Peroxidation in Differentiated C2C12 Myotubes
Abstract
Taurine is a nonproteinogenic amino sulfonic acid in mammals. Interestingly, skeletal muscle is unable to synthesize taurine endogenously, and the processing of muscular taurine changes throughout ageing and under specific pathophysiological conditions, such as muscular dystrophy. Ageing and disease are also associated with altered iron metabolism, especially when there is an excess of labile iron. The present study addresses the question of whether taurine connects cytoprotective effects and redox homeostasis in a previously unknown iron-dependent manner. Using cultured differentiated C2C12 myotubes, the impact of taurine on markers of lipid peroxidation, redox-sensitive enzymes and iron-related proteins was studied. Significant increases in the heme protein myoglobin and the iron storage protein ferritin were observed in response to taurine treatment. Taurine supplementation reduced lipid peroxidation and BODIPY oxidation by ~60 and 25%, respectively. Furthermore, the mRNA levels of redox-sensitive heme oxygenase (Hmox1), catalase (Cat) and glutamate-cysteine ligase (Gclc) and the total cellular glutathione content were lower in taurine-supplemented cells than they were in the control cells. We suggest that taurine may inhibit the initiation and propagation of lipid peroxidation by lowering basal levels of cellular stress, perhaps through reduction of the cellular labile iron pool.
Keywords: BODIPY; glutathione; labile iron pool; myoglobin; skeletal muscle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
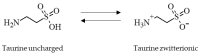
Similar articles
-
Taurine: A Regulator of Cellular Redox Homeostasis and Skeletal Muscle Function.Mol Nutr Food Res. 2019 Aug;63(16):e1800569. doi: 10.1002/mnfr.201800569. Epub 2018 Oct 7. Mol Nutr Food Res. 2019. PMID: 30211983 Review.
-
Moringa oleifera Leaf Extract Upregulates Nrf2/HO-1 Expression and Ameliorates Redox Status in C2C12 Skeletal Muscle Cells.Molecules. 2021 Aug 20;26(16):5041. doi: 10.3390/molecules26165041. Molecules. 2021. PMID: 34443628 Free PMC article.
-
Hyperforin Enhances Heme Oxygenase-1 Expression Triggering Lipid Peroxidation in BRAF-Mutated Melanoma Cells and Hampers the Expression of Pro-Metastatic Markers.Antioxidants (Basel). 2023 Jun 30;12(7):1369. doi: 10.3390/antiox12071369. Antioxidants (Basel). 2023. PMID: 37507910 Free PMC article.
-
Oxidative and glycolytic skeletal muscles deploy protective mechanisms to avoid atrophy under pathophysiological iron overload.J Cachexia Sarcopenia Muscle. 2022 Apr;13(2):1250-1261. doi: 10.1002/jcsm.12897. Epub 2022 Feb 3. J Cachexia Sarcopenia Muscle. 2022. PMID: 35118832 Free PMC article.
-
Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis.Int J Mol Sci. 2022 Dec 27;24(1):449. doi: 10.3390/ijms24010449. Int J Mol Sci. 2022. PMID: 36613888 Free PMC article. Review.
Cited by
-
Effects of dietary rumen-protected glucose level and taurine supplementation on weight change and oxidative stress state of yaks after transport.Front Vet Sci. 2024 Nov 21;11:1492747. doi: 10.3389/fvets.2024.1492747. eCollection 2024. Front Vet Sci. 2024. PMID: 39641099 Free PMC article.
-
Tauroursodeoxycholic Acid (TUDCA)-Lipid Interactions and Antioxidant Properties of TUDCA Studied in Model of Photoreceptor Membranes.Membranes (Basel). 2021 Apr 29;11(5):327. doi: 10.3390/membranes11050327. Membranes (Basel). 2021. PMID: 33946822 Free PMC article.
-
Effects of energy drinks on myogenic differentiation of murine C2C12 myoblasts.Sci Rep. 2023 May 25;13(1):8481. doi: 10.1038/s41598-023-35338-7. Sci Rep. 2023. PMID: 37231025 Free PMC article.
-
Stearidonic acid improves eicosapentaenoic acid status: studies in humans and cultured hepatocytes.Front Nutr. 2024 Apr 4;11:1359958. doi: 10.3389/fnut.2024.1359958. eCollection 2024. Front Nutr. 2024. PMID: 38974810 Free PMC article.
-
Iron Metabolism in the Tumor Microenvironment-Implications for Anti-Cancer Immune Response.Cells. 2021 Feb 2;10(2):303. doi: 10.3390/cells10020303. Cells. 2021. PMID: 33540645 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

