High Dose Ifosfamide in Relapsed and Unresectable High-Grade Osteosarcoma Patients: A Retrospective Series
- PMID: 33142760
- PMCID: PMC7692098
- DOI: 10.3390/cells9112389
High Dose Ifosfamide in Relapsed and Unresectable High-Grade Osteosarcoma Patients: A Retrospective Series
Abstract
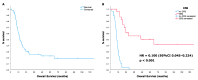
Background: The evidence on high-dose ifosfamide (HD-IFO) use in patients with relapsed osteosarcoma is limited. We performed a retrospective study to analyze HD-IFO activity. Methods: Patients with osteosarcoma relapsed after standard treatment [methotrexate, doxorubicin, cisplatin +/- ifosfamide (MAP+/-I)] with measurable disease according to RECIST1.1 were eligible to ifosfamide (3 g/m2/day) continuous infusion (c.i.) days 1-5 q21d. RECIST1.1 overall response rate (ORR) (complete response (CR) + partial response (PR)), progression-free survival at 6-month (6m-PFS), duration of response (DOR), and 2-year overall survival (2y-OS) were assessed. PARP1 expression and gene mutations were tested by immunohistochemistry and next-generation sequencing. Results: 51 patients were included. ORR was 20% (1 CR + 9 PR). Median DOR was 5 months (95%CI 2-7). Median PFS, 6m-PFS, OS, and 2y-OS were 6 months (95%CI 4-9), 51%, 15 months (10-19), and 30%, respectively. A second surgical complete remission (CR2) was achieved in 26 (51%) patients. After multivariate analysis, previous use of ifosfamide (HR 2.007, p = 0.034) and CR2 (HR 0.126, p < 0.001) showed a significant correlation with PFS and OS, respectively. No significant correlation was found between outcomes and PARP1 or gene mutations. Conclusions: HD-IFO should be considered as the standard first-line treatment option in relapsed osteosarcoma and control arm of future trial in this setting.
Keywords: PARP1; chemotherapy; high-grade bone sarcoma; ifosfamide; osteosarcoma; pediatric bone tumors; sequencing.
Conflict of interest statement
E.P. has served on advisory boards for Amgen, Daiichi Sankyo, Lilly, Deciphera, Eusa Pharma, has received other research support from Bristol Myers Squibb, Pfizer, PharmaMar, Daiichi Sankyo, Incyte, and travel support from Lilly, PharmaMar, and Takeda outside the submitted work. G.G. received honoraria from Bayer, Eisai, Pfizer, Pharmamar, Novartis, has served on advisory boards for Bayer, Eisai, Pfizer, Pharmamar, Novartis, has received research funding from Pharmamar, and travel support from Pharmamar, Tesaro, outside the sumbitted work. L.D. has served on advisory boards for PSI, GSK; performed editorial activity for Novartis; received travel support from Pharmamar, Eli Lilly, Celgene; outside the submitted work. P.P. received institutional funding from Amgen and Pharmamar, outside the sumbitted work. S.F. receved honoraria from Takeda and Pharmamar, outside the sumbitted work.
Figures

References
-
- Campanacci M. Clinical Features, Imaging, Pathology and Treatment. 2nd ed. Springer; New York, NY, USA: 1999. Bone and Soft Tissue Tumors; pp. 463–557.
-
- Fletcher C.D.M., Bridge J.A., Hogendoorn P.C.W., Mertens F. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. IARC Press; Lyon, France: 2013.
-
- Casali P.G., Bielack S., Abecassis N., Aro H.T., Bauer S., Biagini R., Bonvalot S., Boukovinas I., Bovee J.V.M.G., Brennan B., et al. Bone sarcomas: ESMO-PaedCan-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018;29:iv79–iv95. doi: 10.1093/annonc/mdy310. - DOI - PubMed
-
- Bielack S.S., Kempf-Bielack B., Delling G., Exner G.U., Flege S., Helmke K., Kotz R., Salzer-Kuntschik M., Werner M., Winkelmann W., et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J. Clin. Oncol. 2002;20:776–790. doi: 10.1200/JCO.2002.20.3.776. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

