Immunomodulatory Effects of Radiotherapy
- PMID: 33142765
- PMCID: PMC7663574
- DOI: 10.3390/ijms21218151
Immunomodulatory Effects of Radiotherapy
Abstract
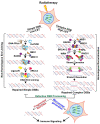
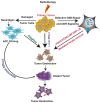
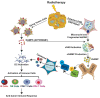
Radiation therapy (RT), an integral component of curative treatment for many malignancies, can be administered via an increasing array of techniques. In this review, we summarize the properties and application of different types of RT, specifically, conventional therapy with x-rays, stereotactic body RT, and proton and carbon particle therapies. We highlight how low-linear energy transfer (LET) radiation induces simple DNA lesions that are efficiently repaired by cells, whereas high-LET radiation causes complex DNA lesions that are difficult to repair and that ultimately enhance cancer cell killing. Additionally, we discuss the immunogenicity of radiation-induced tumor death, elucidate the molecular mechanisms by which radiation mounts innate and adaptive immune responses and explore strategies by which we can increase the efficacy of these mechanisms. Understanding the mechanisms by which RT modulates immune signaling and the key players involved in modulating the RT-mediated immune response will help to improve therapeutic efficacy and to identify novel immunomodulatory drugs that will benefit cancer patients undergoing targeted RT.
Keywords: FLASH-RT; abscopal effects; cancer vaccines; carbon ion therapy; charged particle therapy; clustered DNA damage; immune signaling; radiation therapy; tumor antigens.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Clustered DNA Double-Strand Breaks: Biological Effects and Relevance to Cancer Radiotherapy.Genes (Basel). 2020 Jan 15;11(1):99. doi: 10.3390/genes11010099. Genes (Basel). 2020. PMID: 31952359 Free PMC article. Review.
-
DNA Damage Clustering after Ionizing Radiation and Consequences in the Processing of Chromatin Breaks.Molecules. 2022 Feb 24;27(5):1540. doi: 10.3390/molecules27051540. Molecules. 2022. PMID: 35268641 Free PMC article. Review.
-
Key biological mechanisms involved in high-LET radiation therapies with a focus on DNA damage and repair.Expert Rev Mol Med. 2022 Mar 31;24:e15. doi: 10.1017/erm.2022.6. Expert Rev Mol Med. 2022. PMID: 35357290 Review.
-
Carbon ion radiation and clustered DNA double-strand breaks.Enzymes. 2022;51:117-130. doi: 10.1016/bs.enz.2022.08.008. Epub 2022 Sep 27. Enzymes. 2022. PMID: 36336405
-
Effects of radiation quality and oxygen on clustered DNA lesions and cell death.Radiat Res. 2011 Nov;176(5):587-602. doi: 10.1667/rr2663.1. Epub 2011 Aug 8. Radiat Res. 2011. PMID: 21823972
Cited by
-
Photon- and Proton-Mediated Biological Effects: What Has Been Learned?Life (Basel). 2022 Dec 22;13(1):30. doi: 10.3390/life13010030. Life (Basel). 2022. PMID: 36675979 Free PMC article. Review.
-
Bladder Cancer Treatments in the Age of Personalized Medicine: A Comprehensive Review of Potential Radiosensitivity Biomarkers.Biomark Insights. 2024 Nov 6;19:11772719241297168. doi: 10.1177/11772719241297168. eCollection 2024. Biomark Insights. 2024. PMID: 39512649 Free PMC article. Review.
-
Comparative Analysis of Atezolizumab Plus Bevacizumab and Hepatic Artery Infusion Chemotherapy in Unresectable Hepatocellular Carcinoma: A Multicenter, Propensity Score Study.Cancers (Basel). 2023 Aug 24;15(17):4233. doi: 10.3390/cancers15174233. Cancers (Basel). 2023. PMID: 37686509 Free PMC article.
-
Radiotherapy and breast cancer: finally, an lncRNA perspective on radiosensitivity and radioresistance.Front Oncol. 2024 Sep 13;14:1437542. doi: 10.3389/fonc.2024.1437542. eCollection 2024. Front Oncol. 2024. PMID: 39346726 Free PMC article. Review.
-
A deep dive into radiation keratopathy; Going beyond the current frontierss.Exp Eye Res. 2025 Feb;251:110234. doi: 10.1016/j.exer.2025.110234. Epub 2025 Jan 6. Exp Eye Res. 2025. PMID: 39778671
References
-
- PTCOG Particle Therapy Facilities in a Planning Stage or under Construction. [(accessed on 31 October 2020)]; Available online: http://ptcog.web.psi.ch/newptcentres.html.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

