p-Cymene Complexes of Ruthenium(II) as Antitumor Agents
- PMID: 33142775
- PMCID: PMC7662397
- DOI: 10.3390/molecules25215063
p-Cymene Complexes of Ruthenium(II) as Antitumor Agents
Abstract
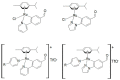
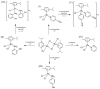
In this work, the cytotoxic behavior of six ruthenium(II) complexes of stoichiometry [(η6-p-cymene)RuCl2L] (I-VI), L = 4-cyanopyridine (I), 2-aminophenol (II), 4-aminophenol (III), pyridazine (IV), and [(η6-p-cymene)RuClL2]PF6; L = cyanopyridine (V), L = 2-aminophenol(VI) towards three cell lines was studied. Two of them, HeLa and MCF-7, are human carcinogenic cells from cervical carcinoma and human breast cancer, respectively. A comparison with healthy cells was carried out with BGM cells which are monkey epithelial cells of renal origin. The behavior of complex II exhibits selectivity towards healthy cells, which is a promising feature for use in cancer treatment since it might reduce the side effects of most current therapies.
Keywords: MTT assay; anticancer activity; complexes; cytotoxicity; ruthenium.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Ruthenium p-Cymene Complexes Incorporating Substituted Pyridine-Quinoline-Based Ligands: Synthesis, Characterization, and Cytotoxic Properties.Molecules. 2024 Jul 6;29(13):3215. doi: 10.3390/molecules29133215. Molecules. 2024. PMID: 38999167 Free PMC article.
-
Improving Cytotoxicity against Breast Cancer Cells by Using Mixed-Ligand Ruthenium(II) Complexes of 2,2'-Bipyridine, Amino Acid, and Nitric Oxide Derivatives as Potential Anticancer Agents.Anticancer Agents Med Chem. 2021;21(12):1602-1611. doi: 10.2174/0929867327666201020155105. Anticancer Agents Med Chem. 2021. PMID: 33081686
-
Effect of N,N Coordination and RuII Halide Bond in Enhancing Selective Toxicity of a Tyramine-Based RuII (p-Cymene) Complex.Inorg Chem. 2020 May 4;59(9):6581-6594. doi: 10.1021/acs.inorgchem.0c00694. Epub 2020 Apr 15. Inorg Chem. 2020. PMID: 32295347
-
GSH-resistant and highly cytoselective ruthenium(II)-p-cymene-(imidazo[4,5-f][1,10]phenanthrolin-2-yl)phenol complexes as potential anticancer agents.Dalton Trans. 2021 Aug 4;50(30):10369-10373. doi: 10.1039/d1dt01604k. Dalton Trans. 2021. PMID: 34308466
-
DNA targeting half sandwich Ru(II)-p-cymene-N^N complexes as cancer cell imaging and terminating agents: influence of regioisomers in cytotoxicity.Dalton Trans. 2021 Jan 27;50(3):979-997. doi: 10.1039/d0dt03107k. Dalton Trans. 2021. PMID: 33355328
Cited by
-
Anti-Inflammatory and Antimicrobial Properties of Thyme Oil and Its Main Constituents.Int J Mol Sci. 2023 Apr 8;24(8):6936. doi: 10.3390/ijms24086936. Int J Mol Sci. 2023. PMID: 37108100 Free PMC article. Review.
-
Myrcene: A Natural Compound Showing Anticancer Activity in HeLa Cells.Molecules. 2023 Sep 21;28(18):6728. doi: 10.3390/molecules28186728. Molecules. 2023. PMID: 37764505 Free PMC article.
-
First-Row Transition Metal Complexes Incorporating the 2-(2'-pyridyl)quinoxaline Ligand (pqx), as Potent Inflammatory Mediators: Cytotoxic Properties and Biological Activities against the Platelet-Activating Factor (PAF) and Thrombin.Molecules. 2023 Oct 1;28(19):6899. doi: 10.3390/molecules28196899. Molecules. 2023. PMID: 37836742 Free PMC article.
-
Synthesis and Characterization of New Ruthenium (II) Complexes of Stoichiometry [Ru(p-Cymene)Cl2L] and Their Cytotoxicity against HeLa-Type Cancer Cells.Molecules. 2022 Oct 26;27(21):7264. doi: 10.3390/molecules27217264. Molecules. 2022. PMID: 36364091 Free PMC article.
-
Age-Related Cancer-Associated Microbiota Potentially Promotes Oral Squamous Cell Cancer Tumorigenesis by Distinct Mechanisms.Front Microbiol. 2022 Apr 15;13:852566. doi: 10.3389/fmicb.2022.852566. eCollection 2022. Front Microbiol. 2022. PMID: 35495663 Free PMC article.
References
-
- Dabrowiak J.C. Metals in Medicine. 2nd ed. John Wiley & Sons Ltd.; Hoboken, NJ, USA: 2017. pp. 91–216.
-
- Schmid W.F., John R.O., Arion V.B., Jakupec M.A., Keppler B.K. Highly Antiproliferative Ruthenium(II) and Osmium(II) Arene Complexes with Paullone-Derived Ligands. Organometallics. 2007;26:6643–6652. doi: 10.1021/om700813c. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

