Promotion of Cellular and Humoral Immunity against Foot-and-Mouth Disease Virus by Immunization with Virus-Like Particles Encapsulated in Monophosphoryl Lipid A and Liposomes
- PMID: 33142799
- PMCID: PMC7712044
- DOI: 10.3390/vaccines8040633
Promotion of Cellular and Humoral Immunity against Foot-and-Mouth Disease Virus by Immunization with Virus-Like Particles Encapsulated in Monophosphoryl Lipid A and Liposomes
Abstract
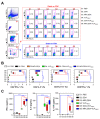
Virus-like particles (VLPs) have emerged as promising vaccine candidates against foot-and-mouth disease (FMD). However, such vaccines provide a relatively low level of protection against FMD virus (FMDV) because of their poor immunogenicity. Therefore, it is necessary to design effective vaccine strategies that induce more potent immunogenicity. In order to investigate the means to improve FMD VLP vaccine (VLPFMDV) immunogenicity, we encapsulated VLPs (MPL/DDA-VLPFMDV) with cationic liposomes based on dimethyldioctadecylammonium bromide (DDA) and/or monophosphoryl lipid A (MPL, TLR4 agonist) as adjuvants. Unlike inactivated whole-cell vaccines, VLPFMDV were successfully encapsulated in this MPL/DDA system. We found that MPL/DDA-VLPFMDV could induce strong cell-mediated immune responses by inducing not only VLP-specific IFN-γ+CD4+ (Th1), IL-17A+CD4+ (Th17), and IFN-γ+CD8+ (activated CD8 response) T cells, but also the development of VLP-specific multifunctional CD4+ and CD8+ memory T cells co-expressing IFN-γ, TNF-α, and IL-2. In addition, the MPL/DDA-VLPFMDV vaccine markedly induced VLP-specific antibody titers; in particular, the vaccine induced greater Th1-predominant IgG responses than VLPFMDV only and DDA-VLPFMDV. These results are expected to provide important clues for the development of an effective VLPFMDV that can induce cellular and humoral immune responses, and address the limitations seen in current VLP vaccines for various diseases.
Keywords: TLR4 agonist; foot-and-mouth disease; immunogenicity; liposome; vaccine; virus-like particles.
Conflict of interest statement
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Salmonella Vaccine Vector System for Foot-and-Mouth Disease Virus and Evaluation of Its Efficacy with Virus-Like Particles.Vaccines (Basel). 2021 Jan 5;9(1):22. doi: 10.3390/vaccines9010022. Vaccines (Basel). 2021. PMID: 33466461 Free PMC article.
-
Incorporation of the TLR4 agonist monophosphoryl lipid A into the bilayer of DDA/TDB liposomes: physico-chemical characterization and induction of CD8+ T-cell responses in vivo.Pharm Res. 2011 Mar;28(3):553-62. doi: 10.1007/s11095-010-0301-9. Epub 2010 Nov 2. Pharm Res. 2011. PMID: 21042837
-
Artificially designed hepatitis B virus core particles composed of multiple epitopes of type A and O foot-and-mouth disease virus as a bivalent vaccine candidate.J Med Virol. 2019 Dec;91(12):2142-2152. doi: 10.1002/jmv.25554. Epub 2019 Aug 24. J Med Virol. 2019. PMID: 31347713
-
Bi-functional gold nanocages enhance specific immunological responses of foot-and-mouth disease virus-like particles vaccine as a carrier and adjuvant.Nanomedicine. 2021 Apr;33:102358. doi: 10.1016/j.nano.2021.102358. Epub 2021 Jan 20. Nanomedicine. 2021. PMID: 33484882
-
Virus-like Particles as Antiviral Vaccine: Mechanism, Design, and Application.Biotechnol Bioprocess Eng. 2023;28(1):1-16. doi: 10.1007/s12257-022-0107-8. Epub 2023 Jan 6. Biotechnol Bioprocess Eng. 2023. PMID: 36627930 Free PMC article. Review.
Cited by
-
B and T Cell Epitopes of the Incursionary Foot-and-Mouth Disease Virus Serotype SAT2 for Vaccine Development.Viruses. 2023 Mar 21;15(3):797. doi: 10.3390/v15030797. Viruses. 2023. PMID: 36992505 Free PMC article. Review.
-
Development of Foot-and-Mouth Disease Vaccines in Recent Years.Vaccines (Basel). 2022 Oct 28;10(11):1817. doi: 10.3390/vaccines10111817. Vaccines (Basel). 2022. PMID: 36366327 Free PMC article. Review.
-
Liposomal delivery system/adjuvant for tuberculosis vaccine.Immun Inflamm Dis. 2023 Jun;11(6):e867. doi: 10.1002/iid3.867. Immun Inflamm Dis. 2023. PMID: 37382263 Free PMC article. Review.
-
Adjuvants: friends in vaccine formulations against infectious diseases.Hum Vaccin Immunother. 2021 Oct 3;17(10):3539-3550. doi: 10.1080/21645515.2021.1934354. Epub 2021 Jul 21. Hum Vaccin Immunother. 2021. PMID: 34288795 Free PMC article. Review.
-
Enhanced Immunogenicity of Foot-and-Mouth Disease Virus-like Particles Using a Water-in-Oil-in-Water Adjuvant.Vaccines (Basel). 2024 Dec 30;13(1):24. doi: 10.3390/vaccines13010024. Vaccines (Basel). 2024. PMID: 39852803 Free PMC article.
References
-
- Lea S., Abu-Ghazaleh R., Blakemore W., Curry S., Fry E., Jackson T., King A., Logan D., Newman J., Stuart D. Structural comparison of two strains of foot-and-mouth disease virus subtype O1 and a laboratory antigenic variant, G67. Structure. 1995;3:571–580. doi: 10.1016/S0969-2126(01)00191-5. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

