The Influence of Soil Acidity on the Physiological Responses of Two Bread Wheat Cultivars
- PMID: 33142829
- PMCID: PMC7692381
- DOI: 10.3390/plants9111472
The Influence of Soil Acidity on the Physiological Responses of Two Bread Wheat Cultivars
Abstract
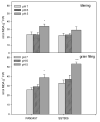
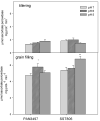
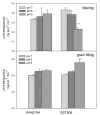
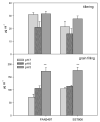
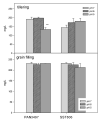
The recent study was conducted to examine the influence of acidic soil on the activities of ascorbate (APX) and guaiacol peroxidase (POD), proline, protein as well as malon-dialdehyde (MDA) content, in two commercial spring wheat cultivars (PAN3497 and SST806) at different growth stages (tillering and grain filling). A cultivar effect was significant only for MDA content, while the treatment effect was highly significant for proline, protein, and MDA. The sampling time effect was significant for most characteristics. MDA, antioxidative capacity, as well as protein content increased with maturity. At grain filling, MDA and proline contents were significantly higher at pH 5 than pH 6 and 7 for both cultivars, with the highest content in SST806. Similarly, SST806 had significantly higher APX and POD when growing at pH 5. There were no significant differences in protein content at grain filling between either genotype or treatments affected by low pH. This study showed that growth stage and soil pH influence the rate of lipid peroxidation as well as the antioxidative capacity of wheat, with a larger effect at grain filling, at pH 5. Although SST806 had higher proline, POD, and APX content than PAN3497 at this growth stage, this coincided with a very high MDA content. This shows that the high antioxidative capacity observed here, was not associated with a reduction of lipid peroxidation under low soil pH. Further research should, therefore, be done to establish the role of the induced antioxidant system in association with growth and yield in wheat.
Keywords: antioxidative enzyme activity; low pH; proline; protein; wheat.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Salicylic Acid Improves Growth and Physiological Attributes and Salt Tolerance Differentially in Two Bread Wheat Cultivars.Plants (Basel). 2022 Jul 15;11(14):1853. doi: 10.3390/plants11141853. Plants (Basel). 2022. PMID: 35890487 Free PMC article.
-
Bread Wheat (Triticum aestivum) Responses to Arbuscular Mycorrhizae Inoculation under Drought Stress Conditions.Plants (Basel). 2021 Aug 24;10(9):1756. doi: 10.3390/plants10091756. Plants (Basel). 2021. PMID: 34579289 Free PMC article.
-
Drought Tolerance Responses in Vegetable-Type Soybean Involve a Network of Biochemical Mechanisms at Flowering and Pod-Filling Stages.Plants (Basel). 2021 Jul 22;10(8):1502. doi: 10.3390/plants10081502. Plants (Basel). 2021. PMID: 34451547 Free PMC article.
-
Ridge-furrow mulched with plastic film improves the anti-oxidative defence system and photosynthesis in leaves of winter wheat under deficit irrigation.PLoS One. 2018 Jul 11;13(7):e0200277. doi: 10.1371/journal.pone.0200277. eCollection 2018. PLoS One. 2018. PMID: 29995903 Free PMC article.
-
Seed priming by sodium nitroprusside improves salt tolerance in wheat (Triticum aestivum L.) by enhancing physiological and biochemical parameters.Plant Physiol Biochem. 2017 Oct;119:50-58. doi: 10.1016/j.plaphy.2017.08.010. Epub 2017 Aug 18. Plant Physiol Biochem. 2017. PMID: 28843888
Cited by
-
Signaling and crosstalk of rhizobacterial and plant hormones that mediate abiotic stress tolerance in plants.Front Microbiol. 2023 Jun 30;14:1171104. doi: 10.3389/fmicb.2023.1171104. eCollection 2023. Front Microbiol. 2023. PMID: 37455718 Free PMC article. Review.
-
The Physiological and Biochemical Responses of European Chestnut (Castanea sativa L.) to Blight Fungus (Cryphonectria parasitica (Murill) Barr).Plants (Basel). 2021 Oct 8;10(10):2136. doi: 10.3390/plants10102136. Plants (Basel). 2021. PMID: 34685944 Free PMC article.
-
Soil acidification and salinity: the importance of biochar application to agricultural soils.Front Plant Sci. 2023 Sep 14;14:1206820. doi: 10.3389/fpls.2023.1206820. eCollection 2023. Front Plant Sci. 2023. PMID: 37780526 Free PMC article. Review.
-
Silicon Nanomaterials Enhance Seedling Growth and Plant Adaptation to Acidic Soil by Promoting Photosynthesis and Antioxidant Activity in Mustard (Brassica campestris L.).Int J Mol Sci. 2024 Sep 25;25(19):10318. doi: 10.3390/ijms251910318. Int J Mol Sci. 2024. PMID: 39408646 Free PMC article.
-
Molecular and Physiological Responses of Citrus sinensis Leaves to Long-Term Low pH Revealed by RNA-Seq Integrated with Targeted Metabolomics.Int J Mol Sci. 2022 May 23;23(10):5844. doi: 10.3390/ijms23105844. Int J Mol Sci. 2022. PMID: 35628662 Free PMC article.
References
-
- Arraiza M.P., Santamarta J.C., Ioras F., García-Rodríguez J.L., Abrudan I.V., Korjas H., Borála G., editors. Climate Change and Restoration of Degraded Land. Colegio de Ingenieros de Montes; Madrid, Spain: 2014.
-
- Schoenholtz S., Miegroet H.V., Burger J. A review of chemical and physiological properties as indicators of forest soil quality. Challenges and opportunities. Ecol. Manag. 2000;138:335–356.
-
- Von Uexkull H.R., Mutert E. Global extent, development and economic impact of acid soils. Plant Soil. 1995;171:1–15. doi: 10.1007/BF00009558. - DOI
-
- Venter A., Herselman J.E., VanderMerwe G.M.E., Steyn C., Beukes D.J. Developing soil acidity maps for South Africa; Proceedings of the 5th International Symposium on Soil Plant Interactions at Low pH; Bergville, South Africa. 12–16 March 2001; p. 21.
-
- Roberts V.G., Smeda Z. The distribution of soil fertility constraints in KwaZulu-Natal, South Africa; Proceedings of the 5th International Symposium on Plant Soil Interactions at low pH; Bergville, South Africa. 12–16 March 2001; p. 12.
LinkOut - more resources
Full Text Sources
Miscellaneous

