Patients with Inflammatory Bowel Disease Are Not at Increased Risk of COVID-19: A Large Multinational Cohort Study
- PMID: 33142843
- PMCID: PMC7693947
- DOI: 10.3390/jcm9113533
Patients with Inflammatory Bowel Disease Are Not at Increased Risk of COVID-19: A Large Multinational Cohort Study
Abstract
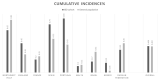
The impact of COVID-19 on inflammatory bowel disease (IBD) patients under pharmacological immunosuppression is still not clearly understood. We investigated the incidence of COVID-19 and the impact of immunosuppression and containment measures on the risk of SARS-CoV-2 infection in a large IBD cohort, from a multicenter cohort from 21st of February to 30th of June, 2020. Ninety-seven patients with IBD (43 UC, 53 CD, one unclassified IBD) and concomitant COVID-19 over a total of 23,879 patients with IBD were enrolled in the study. The cumulative incidence of SARS-CoV-2 infection in patients with IBD vs. the general population was 0.406% and 0.402% cases, respectively. Twenty-three patients (24%) were hospitalized, 21 (22%) had pneumonia, four (4%) were admitted to the Intensive Care Unit, and one patient died. Lethality in our cohort was 1% compared to 9% in the general population. At multivariable analysis, age > 65 years was associated with increased risk of pneumonia and hospitalization (OR 11.6, 95% CI 2.18-62.60; OR 5.1, 95% CI 1.10-23.86, respectively), treatment with corticosteroids increased the risk of hospitalization (OR 7.6, 95% CI 1.48-40.05), whereas monoclonal antibodies were associated with reduced risk of pneumonia and hospitalization (OR 0.1, 95% CI 0.04-0.52; OR 0.3, 95% CI 0.10-0.90, respectively). The risk of COVID-19 in patients with IBD is similar to the general population. National lockdown was effective in preventing infection in our cohort. Advanced age and treatment with corticosteroids impacted negatively on the outcome of COVID-19, whereas monoclonal antibodies did not seem to have a detrimental effect.
Keywords: COVID-19; drugs; immunosuppression; inflammatory bowel disease.
Conflict of interest statement
M.A. received consulting fees from Nikkiso Europe and lecture fees from Janssen, Abbvie and Pfizer; M.C. has served as a speaker or has received research or education funding from MSD, Abbvie, Hospira, Pfizer, Takeda, Janssen, Ferring, Shire Pharmaceuticals, Falk Pharma, Tillotts Pharma; H.A.G. has received lecture fees from Falk Pharma, Pfizer, Takeda, and Nestle; M.B.W. declares educational activities, research projects, scientific meetings, and advisory boards sponsored by MSD, Ferring, Abbvie, Janssen, Biogen and Takeda; U.K. received speaker and advisory fees from ABBVIE, Jannsen, MSD, Takeda, Medtronic. Research support, Jannsen, Takeda, Medtronic; G.B. has received advisory and/or speaker fees and/or research support from AbbVie, Celgene, Ferrirng, Genesis, Janssen, MSD, Mylan, Pfizer, Takeda, Galenica; G.J.M. has received advisory and/or speaker fees and/or research support from AbbVie, Celgene, Celtrion, Ferrirng, Genesis, Hospira, Janssen, Millennium Pharmaceuticals, MSD, Mylan, Pharmacosmos, Pfizer, Takeda, VIANEX, Angelini, Falk Pharma, Galenica, Omega Pharma, Menarini Group, Pharmathen; S.B.H. received advisory and/or research support from Abbvie, MSD, Janssen, Celltrion, Takeda, GSK, Pfizer; L.P.B. reports personal fees from AbbVie, Janssen, Genentech, Ferring, Tillots, Pharmacosmos, Celltrion, Takeda, Boerhinger Ingelheim, Pfizer, Index Pharmaceuticals, Sandoz, Celgene, Biogen, Samsung Bioepis, Alma, Sterna, Nestle, Enterome, Allergan, MSD, Roche, Arena, Gilead, Hikma, Amgen, BMS, Vifor, Norgine; Mylan, Lilly, Fresenius Kabi, Oppilan Pharma, Sublimity Therapeutics, Applied Molecular Transport, OSE Immunotherapeutics, Enthera, Theravance; grants from Abbvie, MSD, Takeda; stock options: CTMA; S.S. holds research grants from Biogen, Takeda, AbbVie, Tillotts Pharma, Ferring and Biohit; served on the advisory boards of Takeda, AbbVie, Merck, Ferring, Pharmacocosmos, Warner Chilcott, Janssen, Falk Pharma, Biohit, TriGenix, Celgene and Tillots Pharma; and has received speaker fees from AbbVie, Biogen, AbbVie, Janssen, Merck, Warner Chilcott and Falk Pharma; J.P.G. has served as a speaker, a consultant, and advisory member for or has received research funding from MSD, Abbvie, Hospira, Pfizer, Kern Pharma, Biogen, Takeda, Janssen, Roche, Sandoz, Celgene, Ferring, Faes Farma, Shire Pharmaceuticals, Falk Pharma, Tillotts Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, and Vifor Pharma; S.D. has served as a speaker, consultant, and advisory board member for Schering-Plough, Abbott (AbbVie) Laboratories, Merck and Co, UCB Pharma, Ferring, Cellerix, Millenium Takeda, Nycomed, Pharmacosmos, Actelion, Alfa Wasserman, Genentech, Grunenthal, Pfizer, Astra Zeneca, Novo Nordisk, Cosmo Pharmaceuticals, Vifor, and Johnson and Johnson; G.F. received consultancy fees from Ferring, MSD, AbbVie, Takeda, Janssen, Amgen, Sandoz, Samsung Bioepis, Celltrion; the other authors have no conflicts to disclose.
Figures
References
-
- John Hopkins University Coronavirus Resource Center Coronavirus Covid-19 Global Cases. [(accessed on 25 October 2020)]; Available online: https://coronavirus.jhu.edu/map.html.
-
- Panaccione R., Löfberg R., Rutgeerts P., Sandborn W.J., Schreiber S., Berg S., Maa J.-F., Petersson J., Robinson A.M., Colombel J.-F. Efficacy and Safety of Adalimumab by Disease Duration: Analysis of Pooled Data From Crohn’s Disease Studies. J. Crohn’s Colitis. 2019;13:725–734. doi: 10.1093/ecco-jcc/jjy223. - DOI - PMC - PubMed
-
- Ng S.C., Shi H.Y., Hamidi N., Underwood F.E., Tang W., Benchimol E.I., Panaccione R., Ghosh S., Wu J.C.Y., Chan F.K.L., et al. Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population-Based Studies. Lancet. 2018;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous