Protective Effects of a Discontinuous Treatment with Alpha-Lipoic Acid in Obesity-Related Heart Failure with Preserved Ejection Fraction, in Rats
- PMID: 33142857
- PMCID: PMC7693016
- DOI: 10.3390/antiox9111073
Protective Effects of a Discontinuous Treatment with Alpha-Lipoic Acid in Obesity-Related Heart Failure with Preserved Ejection Fraction, in Rats
Abstract
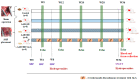
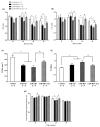
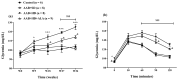
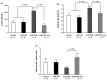
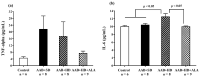
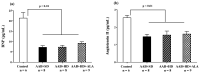
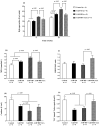
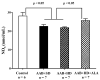
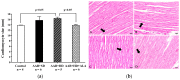
Obesity induces hemodynamic and humoral changes that are associated with functional and structural cardiac remodeling, which ultimately result in the development of heart failure (HF) with preserved ejection fraction (HFpEF). In recent years, pharmacological studies in patients with HFpEF were mostly unsatisfactory. In these conditions, alternative new therapeutic approaches are necessary. The aim of our study was (1) to assess the effects of obesity on heart function in an experimental model and (2) to evaluate the efficacy of an alpha-lipoic acid (ALA) antioxidant treatment. Sprague-Dawley rats (7 weeks old) were either included in the control group (n = 6) or subjected to abdominal aortic banding (AAB) and divided into three subgroups, depending on their diet: standard (AAB + SD, n = 8), hypecaloric (AAB + HD, n = 8) and hypercaloric with discontinuous ALA treatment (AAB + HD + ALA, n = 9). Body weight (BW), glycemia, echocardiography parameters and plasma hydroperoxides were monitored throughout the study. After 36 weeks, plasma adiposity (leptin and adiponectin) and inflammation (IL-6 and TNF-alpha) markers, together with B-type natriuretic peptide and oxidative stress markers (end-products of lipid peroxidation and endogenous antioxidant systems) were assessed. Moreover, cardiac fiber diameters were measured. In our experiment, diet-induced obesity generated cardiometabolic disturbances, and in association with pressure-overload induced by AAB, it precipitated the onset of heart failure, cardiac hypertrophy and diastolic dysfunction, while producing a pro-oxidant and pro-inflammatory plasmatic status. In relationship with its antioxidant effects, the chronic ALA-discontinuous treatment prevented BW gain and decreased metabolic and cardiac perturbations, confirming its protective effects on the cardiovascular system.
Keywords: alpha-lipoic acid; antioxidants; heart failure with preserved ejection fraction; obesity; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S., Falk V., González-Juanatey J.R., Harjola V.P., Jankowska E.A., et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

