Downregulation of CTRP-3 by Weight Loss In Vivo and by Bile Acids and Incretins in Adipocytes In Vitro
- PMID: 33142914
- PMCID: PMC7662344
- DOI: 10.3390/ijms21218168
Downregulation of CTRP-3 by Weight Loss In Vivo and by Bile Acids and Incretins in Adipocytes In Vitro
Abstract
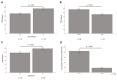
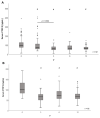
The adipokine CTRP-3 (C1q/TNF-related protein-3) exerts anti-inflammatory and anti-diabetic effects. Its regulation in obesity and during weight loss is unknown. Serum and adipose tissue (AT) samples were obtained from patients (n = 179) undergoing bariatric surgery (BS). Moreover, patients (n = 131) participating in a low-calorie diet (LCD) program were studied. CTRP 3 levels were quantified by ELISA and mRNA expression was analyzed in AT and in 3T3-L1 adipocytes treated with bile acids and incretins. There was a persistent downregulation of CTRP-3 serum levels during weight loss. CTRP-3 expression was higher in subcutaneous than in visceral AT and serum levels of CTRP-3 were positively related to AT expression levels. A rapid decrease of circulating CTRP-3 was observed immediately upon BS, suggesting weight loss-independent regulatory mechanisms. Adipocytes CTRP-3 expression was inhibited by primary bile acid species and GLP 1. Adipocyte-specific CTRP-3 deficiency increased bile acid receptor expression. Circulating CTRP-3 levels are downregulated during weight loss, with a considerable decline occurring immediately upon BS. Mechanisms dependent and independent of weight loss cause the post-surgical decline of CTRP-3. The data strongly argue for regulatory interrelations of CTRP-3 with bile acids and incretin system.
Keywords: C1q/TNF-related protein-3; bariatric surgery; bile acids; incretins; low calorie diet; obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Wong G.W., Krawczyk S.A., Kitidis-Mitrokostas C., Revett T., Gimeno R., Lodish H.F. Molecular, biochemical and functional characterizations of C1q/TNF family members: Adipose-tissue-selective expression patterns, regulation by PPAR-γ agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem. J. 2008;416:161–177. doi: 10.1042/BJ20081240. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

