Membrane-Targeting Triphenylphosphonium Functionalized Ciprofloxacin for Methicillin-Resistant Staphylococcus aureus (MRSA)
- PMID: 33143023
- PMCID: PMC7693559
- DOI: 10.3390/antibiotics9110758
Membrane-Targeting Triphenylphosphonium Functionalized Ciprofloxacin for Methicillin-Resistant Staphylococcus aureus (MRSA)
Abstract
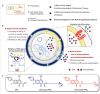
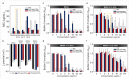
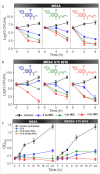
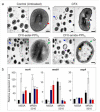
Multidrug-resistant (MDR) bacteria have become a severe problem for public health. Developing new antibiotics for MDR bacteria is difficult, from inception to the clinically approved stage. Here, we have used a new approach, modification of an antibiotic, ciprofloxacin (CFX), with triphenylphosphonium (TPP, PPh3) moiety via ester- (CFX-ester-PPh3) and amide-coupling (CFX-amide-PPh3) to target bacterial membranes. In this study, we have evaluated the antibacterial activities of CFX and its derivatives against 16 species of bacteria, including MDR bacteria, using minimum inhibitory concentration (MIC) assay, morphological monitoring, and expression of resistance-related genes. TPP-conjugated CFX, CFX-ester-PPh3, and CFX-amide-PPh3 showed significantly improved antibacterial activity against Gram-positive bacteria, Staphylococcus aureus, including MDR S. aureus (methicillin-resistant S. aureus (MRSA)) strains. The MRSA ST5 5016 strain showed high antibacterial activity, with MIC values of 11.12 µg/mL for CFX-ester-PPh3 and 2.78 µg/mL for CFX-amide-PPh3. The CFX derivatives inhibited biofilm formation in MRSA by more than 74.9% of CFX-amide-PPh3. In the sub-MIC, CFX derivatives induced significant morphological changes in MRSA, including irregular deformation and membrane disruption, accompanied by a decrease in the level of resistance-related gene expression. With these promising results, this method is very likely to combat MDR bacteria through a simple TPP moiety modification of known antibiotics, which can be readily prepared at clinical sites.
Keywords: antibiotic conjugates; ciprofloxacin; multidrug resistance bacteria; triphenyl-phosphonium.
Conflict of interest statement
The authors declare the following competing financial interest(s): the authors are listed as inventors on a pending patent application related to technology described in this work.
Figures
Similar articles
-
Development of membrane-targeting TPP+-chloramphenicol conjugates to combat methicillin-resistant staphylococcus aureus (MRSA) infections.Eur J Med Chem. 2024 Jan 15;264:115973. doi: 10.1016/j.ejmech.2023.115973. Epub 2023 Nov 27. Eur J Med Chem. 2024. PMID: 38096652
-
Determination of antibacterial activity and minimum inhibitory concentration of larval extract of fly via resazurin-based turbidometric assay.BMC Microbiol. 2017 Feb 16;17(1):36. doi: 10.1186/s12866-017-0936-3. BMC Microbiol. 2017. PMID: 28209130 Free PMC article.
-
Synthesis and evaluation of new quinazolin-4(3H)-one derivatives as potent antibacterial agents against multidrug resistant Staphylococcus aureus and Mycobacterium tuberculosis.Eur J Med Chem. 2019 Aug 1;175:287-308. doi: 10.1016/j.ejmech.2019.04.067. Epub 2019 Apr 28. Eur J Med Chem. 2019. PMID: 31096152
-
Terminalia bellirica fruit extracts: in-vitro antibacterial activity against selected multidrug-resistant bacteria, radical scavenging activity and cytotoxicity study on BHK-21 cells.BMC Complement Altern Med. 2018 Dec 7;18(1):325. doi: 10.1186/s12906-018-2382-7. BMC Complement Altern Med. 2018. PMID: 30526562 Free PMC article.
-
Evaluation of membrane fluidity of multidrug-resistant isolates of Escherichia coli and Staphylococcus aureus in presence and absence of antibiotics.J Photochem Photobiol B. 2018 Apr;181:150-156. doi: 10.1016/j.jphotobiol.2018.03.002. Epub 2018 Mar 5. J Photochem Photobiol B. 2018. PMID: 29567316
Cited by
-
Triphenilphosphonium Analogs of Chloramphenicol as Dual-Acting Antimicrobial and Antiproliferating Agents.Antibiotics (Basel). 2021 Apr 23;10(5):489. doi: 10.3390/antibiotics10050489. Antibiotics (Basel). 2021. PMID: 33922611 Free PMC article.
-
Rhodamine 19 Alkyl Esters as Effective Antibacterial Agents.Int J Mol Sci. 2024 Jun 2;25(11):6137. doi: 10.3390/ijms25116137. Int J Mol Sci. 2024. PMID: 38892325 Free PMC article.
-
Alkyltriphenylphosphonium-Functionalized Hyperbranched Polyethyleneimine Nanoparticles for Safe and Efficient Bacterial Eradication: A Structure-Property Relationship Study.Int J Mol Sci. 2025 May 28;26(11):5153. doi: 10.3390/ijms26115153. Int J Mol Sci. 2025. PMID: 40507972 Free PMC article.
-
Integration of Transcriptomics and Metabolomics Reveals the Antitumor Mechanism of Protopanaxadiol Triphenylphosphate Derivative in Non-Small-Cell Lung Cancer.Molecules. 2024 Sep 9;29(17):4275. doi: 10.3390/molecules29174275. Molecules. 2024. PMID: 39275122 Free PMC article.
-
Can Porphyrin-Triphenylphosphonium Conjugates Enhance the Photosensitizer Performance Toward Bacterial Strains?ACS Appl Bio Mater. 2024 Aug 19;7(8):5541-5552. doi: 10.1021/acsabm.4c00659. Epub 2024 Jul 15. ACS Appl Bio Mater. 2024. PMID: 39008849 Free PMC article.
References
-
- Alam M.M., Islam M., Wahab A., Billah M. Antimicrobial resistance crisis and combating approaches. J. Med. 2019;20:38–45. doi: 10.3329/jom.v20i1.38842. - DOI
Grants and funding
- NRF-2019-M3A9H1103783/National Research Foundation of Korea
- NRF-2018-M3A9H3021707/National Research Foundation of Korea
- NRF-2018-R1A6A1A03025124/National Research Foundation of Korea
- NRF-2018-R1D1A1B07043383/National Research Foundation of Korea
- NRF-2018-R1C1B5043292/National Research Foundation of Korea
LinkOut - more resources
Full Text Sources

