Recent Advances in Molecular Docking for the Research and Discovery of Potential Marine Drugs
- PMID: 33143025
- PMCID: PMC7692358
- DOI: 10.3390/md18110545
Recent Advances in Molecular Docking for the Research and Discovery of Potential Marine Drugs
Abstract
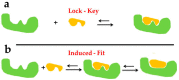
Marine drugs have long been used and exhibit unique advantages in clinical practices. Among the marine drugs that have been approved by the Food and Drug Administration (FDA), the protein-ligand interactions, such as cytarabine-DNA polymerase, vidarabine-adenylyl cyclase, and eribulin-tubulin complexes, are the important mechanisms of action for their efficacy. However, the complex and multi-targeted components in marine medicinal resources, their bio-active chemical basis, and mechanisms of action have posed huge challenges in the discovery and development of marine drugs so far, which need to be systematically investigated in-depth. Molecular docking could effectively predict the binding mode and binding energy of the protein-ligand complexes and has become a major method of computer-aided drug design (CADD), hence this powerful tool has been widely used in many aspects of the research on marine drugs. This review introduces the basic principles and software of the molecular docking and further summarizes the applications of this method in marine drug discovery and design, including the early virtual screening in the drug discovery stage, drug target discovery, potential mechanisms of action, and the prediction of drug metabolism. In addition, this review would also discuss and prospect the problems of molecular docking, in order to provide more theoretical basis for clinical practices and new marine drug research and development.
Keywords: marine drugs; mechanism of action; molecular docking; protein–ligand interaction; target protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Computational Methodologies in the Exploration of Marine Natural Product Leads.Mar Drugs. 2018 Jul 13;16(7):236. doi: 10.3390/md16070236. Mar Drugs. 2018. PMID: 30011882 Free PMC article. Review.
-
Natural products used as a chemical library for protein-protein interaction targeted drug discovery.J Mol Graph Model. 2018 Jan;79:46-58. doi: 10.1016/j.jmgm.2017.10.015. Epub 2017 Oct 27. J Mol Graph Model. 2018. PMID: 29136547
-
Predicting Antifouling Activity and Acetylcholinesterase Inhibition of Marine-Derived Compounds Using a Computer-Aided Drug Design Approach.Mar Drugs. 2022 Feb 8;20(2):129. doi: 10.3390/md20020129. Mar Drugs. 2022. PMID: 35200658 Free PMC article.
-
Synthesis of Marine Natural Products and Molecules Inspired by Marine Substances.Mar Drugs. 2021 Apr 8;19(4):208. doi: 10.3390/md19040208. Mar Drugs. 2021. PMID: 33917783 Free PMC article.
-
Overview on the current status on virtual high-throughput screening and combinatorial chemistry approaches in multi-target anticancer drug discovery; Part II.J BUON. 2016 Nov-Dec;21(6):1337-1358. J BUON. 2016. PMID: 28039691 Review.
Cited by
-
Synthesis and Evaluation of Antiproliferative Activity, Topoisomerase IIα Inhibition, DNA Binding and Non-Clinical Toxicity of New Acridine-Thiosemicarbazone Derivatives.Pharmaceuticals (Basel). 2022 Sep 2;15(9):1098. doi: 10.3390/ph15091098. Pharmaceuticals (Basel). 2022. PMID: 36145320 Free PMC article.
-
Production of Fucoxanthin from Microalgae Isochrysis galbana of Djibouti: Optimization, Correlation with Antioxidant Potential, and Bioinformatics Approaches.Mar Drugs. 2024 Aug 6;22(8):358. doi: 10.3390/md22080358. Mar Drugs. 2024. PMID: 39195473 Free PMC article.
-
Potential Anti-aging Components From Moringa oleifera Leaves Explored by Affinity Ultrafiltration With Multiple Drug Targets.Front Nutr. 2022 May 10;9:854882. doi: 10.3389/fnut.2022.854882. eCollection 2022. Front Nutr. 2022. PMID: 35619958 Free PMC article.
-
Network Pharmacology Prediction and Molecular Docking-Based Strategy to Explore the Potential Mechanism of Gualou Xiebai Banxia Decoction against Myocardial Infarction.Genes (Basel). 2024 Mar 22;15(4):392. doi: 10.3390/genes15040392. Genes (Basel). 2024. PMID: 38674327 Free PMC article.
-
Using Network Pharmacology and Molecular Docking to Explore the Mechanism of Shan Ci Gu (Cremastra appendiculata) Against Non-Small Cell Lung Cancer.Front Chem. 2021 Jun 9;9:682862. doi: 10.3389/fchem.2021.682862. eCollection 2021. Front Chem. 2021. PMID: 34178945 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

