Intramolecular Lactones of Sialic Acids
- PMID: 33143039
- PMCID: PMC7663150
- DOI: 10.3390/ijms21218098
Intramolecular Lactones of Sialic Acids
Abstract
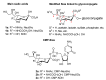
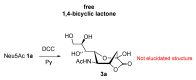
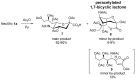
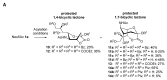
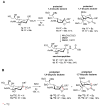
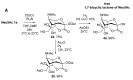
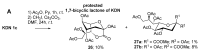
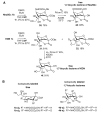
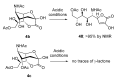
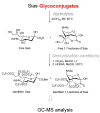
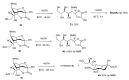
The so-called "sialo-chemical-biology" has become an attractive research area, as an increasing number of natural products containing a sialic acid moiety have been shown to play important roles in biological, pathological, and immunological processes. The intramolecular lactones of sialic acids are a subclass from this crucial family that could have central functions in the discrimination of physiological and pathological conditions. In this review, we report an in-depth analysis of the synthetic achievements in the preparation of the intramolecular lactones of sialic acids (1,4-, 1,7- and γ-lactones), in their free and/or protected form. In particular, recent advances in the synthesis of the 1,7-lactones have allowed the preparation of key sialic acid derivatives. These compounds could be used as authentic reference standards for their correct determination in biological samples, thus overcoming some of the limitations of the previous analytical procedures.
Keywords: biomarker; heptafluoro derivatives; lactone; sialic acid; tumor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






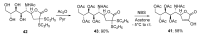
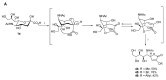

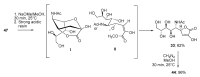
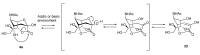
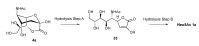
References
-
- Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., Darvill A.G., Kinoshita T., Packer N.H., Prestegard J.H., et al. Essentials of Glycobiology. 3rd ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 2015. - PubMed

