Aging-Affected MSC Functions and Severity of Periodontal Tissue Destruction in a Ligature-Induced Mouse Periodontitis Model
- PMID: 33143068
- PMCID: PMC7663404
- DOI: 10.3390/ijms21218103
Aging-Affected MSC Functions and Severity of Periodontal Tissue Destruction in a Ligature-Induced Mouse Periodontitis Model
Abstract
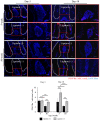
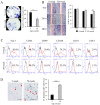
Mesenchymal stem cells (MSCs) are known to play important roles in the repair of lost or damaged tissues and immunotolerance. On the other hand, aging is known to impair MSC function. However, little is currently known about how aged MSCs affect the host response to the local inflammatory condition and tissue deterioration in periodontitis, which is a progressive destructive disease of the periodontal tissue potentially leading to multiple tooth loss. In this study, we examined the relationship between aging-induced impairment of MSC function and the severity of periodontal tissue destruction associated with the decrease in host immunomodulatory response using a ligature-induced periodontitis model in young and aged mice. The results of micro computerized tomography (micro-CT) and histological analysis revealed a more severe bone loss associated with increased osteoclast activity in aged (50-week-old) mice compared to young (5-week-old) mice. Immunostaining analysis revealed that, in aged mice, the accumulation of inflammatory T and B cells was higher, whereas the percentage of platelet-derived growth factor receptor α (PDGFRα)+ MSCs, which are known to modulate the apoptosis of T cells, was significantly lower than in young mice. In vitro analysis of MSC function showed that the expression of surface antigen markers for MSCs (Sca-1, CD90, CD146), colony formation, migration, and osteogenic differentiation of aged MSCs were significantly declined compared to those of young MSCs. Moreover, a significantly higher proportion of aged MSCs were positive for the senescence-associated β galactosidase activity. Importantly, aged MSCs presented a decreased expression of FAS-L, which was associated with a lower immunomodulatory property of aged MSCs to induce T cell apoptosis in co-cultures compared with young MSCs. In summary, this is the first study showing that aging-induced impairment of MSC function, including immunomodulatory response, is potentially correlated with progressive periodontal tissue deterioration.
Keywords: aging; bone resorption; immunomodulation; mesenchymal stem cell; periodontitis; tissue destruction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Neutrophils suppress osteogenic differentiation of Gli1+ stem cells via neutrophil extracellular traps and contribute to bone loss in periodontitis.Biochem Biophys Res Commun. 2024 Dec 10;737:150916. doi: 10.1016/j.bbrc.2024.150916. Epub 2024 Oct 28. Biochem Biophys Res Commun. 2024. PMID: 39489114
-
Regenerative Potential of Bone Morphogenetic Protein 7-Engineered Mesenchymal Stem Cells in Ligature-Induced Periodontitis.Tissue Eng Part A. 2023 Apr;29(7-8):200-210. doi: 10.1089/ten.TEA.2022.0162. Epub 2023 Feb 23. Tissue Eng Part A. 2023. PMID: 36565024
-
Local application of IGFBP5 protein enhanced periodontal tissue regeneration via increasing the migration, cell proliferation and osteo/dentinogenic differentiation of mesenchymal stem cells in an inflammatory niche.Stem Cell Res Ther. 2017 Sep 29;8(1):210. doi: 10.1186/s13287-017-0663-6. Stem Cell Res Ther. 2017. PMID: 28962660 Free PMC article.
-
Advances in mesenchymal stem cell conditioned medium-mediated periodontal tissue regeneration.J Transl Med. 2021 Nov 4;19(1):456. doi: 10.1186/s12967-021-03125-5. J Transl Med. 2021. PMID: 34736500 Free PMC article. Review.
-
Immunomodulatory and potential therapeutic role of mesenchymal stem cells in periodontitis.J Physiol Pharmacol. 2014 Jun;65(3):327-39. J Physiol Pharmacol. 2014. PMID: 24930504 Review.
Cited by
-
miR-21 upregulation exacerbates pressure overload-induced cardiac hypertrophy in aged hearts.Aging (Albany NY). 2022 Jul 28;14(14):5925-5945. doi: 10.18632/aging.204194. Epub 2022 Jul 28. Aging (Albany NY). 2022. PMID: 35907209 Free PMC article.
-
Autophagy in aging-related oral diseases.Front Endocrinol (Lausanne). 2022 Aug 5;13:903836. doi: 10.3389/fendo.2022.903836. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35992149 Free PMC article. Review.
-
The Emergence of Senescent Surface Biomarkers as Senotherapeutic Targets.Cells. 2021 Jul 9;10(7):1740. doi: 10.3390/cells10071740. Cells. 2021. PMID: 34359910 Free PMC article. Review.
-
Cellular Senescence and Periodontitis: Mechanisms and Therapeutics.Biology (Basel). 2022 Sep 29;11(10):1419. doi: 10.3390/biology11101419. Biology (Basel). 2022. PMID: 36290323 Free PMC article. Review.
-
Therapeutic effects of peptide P140 in a mouse periodontitis model.Cell Mol Life Sci. 2022 Sep 15;79(10):518. doi: 10.1007/s00018-022-04537-2. Cell Mol Life Sci. 2022. PMID: 36104457 Free PMC article.
References
-
- World Health Organization International Statistical Classification of Diseases and Related Health Problems. [(accessed on 23 October 2020)]; Available online: https://icd.who.int/
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

