Transcriptional Analysis of Carotenoids Accumulation and Metabolism in a Pink-Fleshed Lemon Mutant
- PMID: 33143225
- PMCID: PMC7692314
- DOI: 10.3390/genes11111294
Transcriptional Analysis of Carotenoids Accumulation and Metabolism in a Pink-Fleshed Lemon Mutant
Abstract
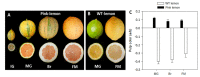
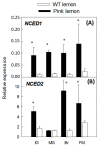
Pink lemon is a spontaneous bud mutation of lemon (Citrus limon, L. Burm. f) characterized by the production of pink-fleshed fruits due to an unusual accumulation of lycopene. To elucidate the genetic determinism of the altered pigmentation, comparative carotenoid profiling and transcriptional analysis of both the genes involved in carotenoid precursors and metabolism, and the proteins related to carotenoid-sequestering structures were performed in pink-fleshed lemon and its wild-type. The carotenoid profile of pink lemon pulp is characterized by an increased accumulation of linear carotenoids, such as lycopene, phytoene and phytofluene, from the early stages of development, reaching their maximum in mature green fruits. The distinctive phenotype of pink lemon is associated with an up-regulation and down-regulation of the genes upstream and downstream the lycopene cyclase, respectively. In particular, 9-cis epoxycarotenoid dioxygenase genes were overexpressed in pink lemon compared with the wild-type, suggesting an altered regulation of abscisic acid biosynthesis. Similarly, during early development of the fruits, genes of the carotenoid-associated proteins heat shock protein 21, fibrillin 1 and 2 and orange gene were overexpressed in the pulp of the pink-fleshed lemon compared to the wild-type, indicating its increased capacity for sequestration, stabilization or accumulation of carotenes. Altogether, the results highlighted significant differences at the transcriptomic level between the pink-fleshed lemon and its wild-type, in terms of carotenoid metabolism and the capacity of stabilization in storage structures between the two accessions. Such changes may be either responsible for the altered carotenoid accumulation or in contrast, a metabolic consequence.
Keywords: Citrus limon; carotenoids; citrus; fruit quality; gene expression; lycopene; pigments.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
A comprehensive analysis of carotenoids metabolism in two red-fleshed mutants of Navel and Valencia sweet oranges (Citrus sinensis).Front Plant Sci. 2022 Oct 18;13:1034204. doi: 10.3389/fpls.2022.1034204. eCollection 2022. Front Plant Sci. 2022. PMID: 36330241 Free PMC article.
-
Accumulation of carotenoids and expression of carotenoid biosynthetic genes during maturation in citrus fruit.Plant Physiol. 2004 Feb;134(2):824-37. doi: 10.1104/pp.103.031104. Epub 2004 Jan 22. Plant Physiol. 2004. PMID: 14739348 Free PMC article.
-
Exploring the diversity in Citrus fruit colouration to decipher the relationship between plastid ultrastructure and carotenoid composition.Planta. 2015 Sep;242(3):645-61. doi: 10.1007/s00425-015-2370-9. Epub 2015 Jul 23. Planta. 2015. PMID: 26202736
-
Metabolic Profiling and Transcriptional Analysis of Carotenoid Accumulation in a Red-Fleshed Mutant of Pummelo (Citrus grandis).Molecules. 2022 Jul 19;27(14):4595. doi: 10.3390/molecules27144595. Molecules. 2022. PMID: 35889470 Free PMC article.
-
Regulation of Carotenoid Biosynthesis During Fruit Development.Subcell Biochem. 2016;79:161-98. doi: 10.1007/978-3-319-39126-7_6. Subcell Biochem. 2016. PMID: 27485222 Review.
Cited by
-
A comprehensive analysis of carotenoids metabolism in two red-fleshed mutants of Navel and Valencia sweet oranges (Citrus sinensis).Front Plant Sci. 2022 Oct 18;13:1034204. doi: 10.3389/fpls.2022.1034204. eCollection 2022. Front Plant Sci. 2022. PMID: 36330241 Free PMC article.
-
ORANGE family proteins: multifunctional chaperones shaping plant carotenoid level, plastid development, stress tolerance, and more.Mol Hortic. 2025 May 9;5(1):43. doi: 10.1186/s43897-025-00169-9. Mol Hortic. 2025. PMID: 40341160 Free PMC article. Review.
-
Comparative transcriptomics of wild and commercial Citrus during early ripening reveals how domestication shaped fruit gene expression.BMC Plant Biol. 2022 Mar 17;22(1):123. doi: 10.1186/s12870-022-03509-9. BMC Plant Biol. 2022. PMID: 35300613 Free PMC article.
-
A dual sgRNA-directed CRISPR/Cas9 construct for editing the fruit-specific β-cyclase 2 gene in pigmented citrus fruits.Front Plant Sci. 2022 Dec 13;13:975917. doi: 10.3389/fpls.2022.975917. eCollection 2022. Front Plant Sci. 2022. PMID: 36582639 Free PMC article.
-
Down-regulation of NCED leads to the accumulation of carotenoids in the flesh of F1 generation of peach hybrid.Front Plant Sci. 2022 Nov 3;13:1055779. doi: 10.3389/fpls.2022.1055779. eCollection 2022. Front Plant Sci. 2022. PMID: 36407629 Free PMC article.
References
-
- Rodriguez-Concepcion M., Avalos J., Bonet M.L., Boronat A., Gomez-Gomez L., Hornero-Mendez D., Limon M.C., Meléndez-Martínez A.J., Olmedilla-Alonso B., Palou A., et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018;70:62–93. doi: 10.1016/j.plipres.2018.04.004. - DOI - PubMed
-
- Rodrigo M.J., Alquézar B., Alós E., Lado J., Zacarías L. Biochemical bases and molecular regulation of pigmentation in the peel of Citrus fruit. Sci. Hortic. 2013;163:46–62. doi: 10.1016/j.scienta.2013.08.014. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

