Processed Eggshell Membrane Powder Is a Promising Biomaterial for Use in Tissue Engineering
- PMID: 33143232
- PMCID: PMC7663119
- DOI: 10.3390/ijms21218130
Processed Eggshell Membrane Powder Is a Promising Biomaterial for Use in Tissue Engineering
Abstract
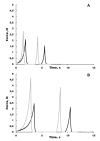
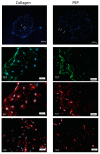
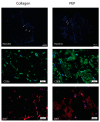
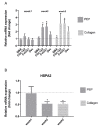
The purpose of this study was to investigate the tissue regenerating and biomechanical properties of processed eggshell membrane powder (PEP) for use in 3D-scaffolds. PEP is a low-cost, natural biomaterial with beneficial bioactive properties. Most importantly, this material is available as a by-product of the chicken egg processing (breaking) industry on a large scale, and it could have potential as a low-cost ingredient for therapeutic scaffolds. Scaffolds consisting of collagen alone and collagen combined with PEP were produced and analyzed for their mechanical properties and the growth of primary fibroblasts and skeletal muscle cells. Mechanical testing revealed that a PEP/collagen-based scaffold increased the mechanical hardness of the scaffold compared with a pure collagen scaffold. Scanning electron microscopy (SEM) demonstrated an interconnected porous structure for both scaffolds, and that the PEP was evenly distributed in dense clusters within the scaffold. Fibroblast and skeletal muscle cells attached, were viable and able to proliferate for 1 and 2 weeks in both scaffolds. The cell types retained their phenotypic properties expressing phenotype markers of fibroblasts (TE7, alpha-smooth muscle actin) and skeletal muscle (CD56) visualized by immunostaining. mRNA expression of the skeletal muscle markers myoD, myogenin, and fibroblasts marker (SMA) together with extracellular matrix components supported viable phenotypes and matrix-producing cells in both types of scaffolds. In conclusion, PEP is a promising low-cost, natural biomaterial for use in combination with collagen as a scaffold for 3D-tissue engineering to improve the mechanical properties and promote cellular adhesion and growth of regenerating cells.
Keywords: bovine muscle cells; cell migration; extracellular matrix; human dermal fibroblasts; myofibroblast; processed eggshell membrane powder; scaffold.
Conflict of interest statement
The authors have the following financial competing interests: The author H.P.S., P.B. and R.S. is employed by Biovotec AS, a biotech company aiming to develop wound treatment products. Biovotec has filed a patent on the use of micronized eggshell (WO2016066718: Micronized eggshell membrane particles and the use thereof to promote the healing of wounds). Micronized eggshell membrane, PEP was provided from Biovotec to Nofima for its research activities. Micronized eggshell particles are not commercialized and are today used for research purposes only. This does not alter our adherence to Biomaterials Science policies on sharing data and materials.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

