Cannabidiol (CBD) as a Promising Anti-Cancer Drug
- PMID: 33143283
- PMCID: PMC7693730
- DOI: 10.3390/cancers12113203
Cannabidiol (CBD) as a Promising Anti-Cancer Drug
Abstract
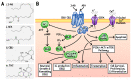
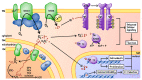
Recently, cannabinoids, such as cannabidiol (CBD) and Δ9 -tetrahydrocannabinol (THC), have been the subject of intensive research and heavy scrutiny. Cannabinoids encompass a wide array of organic molecules, including those that are physiologically produced in humans, synthesized in laboratories, and extracted primarily from the Cannabis sativa plant. These organic molecules share similarities in their chemical structures as well as in their protein binding profiles. However, pronounced differences do exist in their mechanisms of action and clinical applications, which will be briefly compared and contrasted in this review. The mechanism of action of CBD and its potential applications in cancer therapy will be the major focus of this review article.
Keywords: CBD; Cannabidiol; Cannbinoids; anti-cancer drug.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

