Increasing Prevalence of HIV-1 Transmitted Drug Resistance in Portugal: Implications for First Line Treatment Recommendations
- PMID: 33143301
- PMCID: PMC7693025
- DOI: 10.3390/v12111238
Increasing Prevalence of HIV-1 Transmitted Drug Resistance in Portugal: Implications for First Line Treatment Recommendations
Abstract
Introduction: Treatment for All recommendations have allowed access to antiretroviral (ARV) treatment for an increasing number of patients. This minimizes the transmission of infection but can potentiate the risk of transmitted (TDR) and acquired drug resistance (ADR).
Objective: To study the trends of TDR and ADR in patients followed up in Portuguese hospitals between 2001 and 2017.
Methods: In total, 11,911 patients of the Portuguese REGA database were included. TDR was defined as the presence of one or more surveillance drug resistance mutation according to the WHO surveillance list. Genotypic resistance to ARV was evaluated with Stanford HIVdb v7.0. Patterns of TDR, ADR and the prevalence of mutations over time were analyzed using logistic regression.
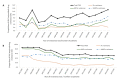
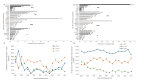
Results and discussion: The prevalence of TDR increased from 7.9% in 2003 to 13.1% in 2017 (p < 0.001). This was due to a significant increase in both resistance to nucleotide reverse transcriptase inhibitors (NRTIs) and non-nucleotide reverse transcriptase inhibitors (NNRTIs), from 5.6% to 6.7% (p = 0.002) and 2.9% to 8.9% (p < 0.001), respectively. TDR was associated with infection with subtype B, and with lower viral load levels (p < 0.05). The prevalence of ADR declined from 86.6% in 2001 to 51.0% in 2017 (p < 0.001), caused by decreasing drug resistance to all antiretroviral (ARV) classes (p < 0.001).
Conclusions: While ADR has been decreasing since 2001, TDR has been increasing, reaching a value of 13.1% by the end of 2017. It is urgently necessary to develop public health programs to monitor the levels and patterns of TDR in newly diagnosed patients.
Keywords: HIV-1; Portugal; acquired drug resistance; transmitted drug resistance.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures

References
-
- 90-90-90: Treatment for All. [(accessed on 23 September 2020)]; Available online: https://www.unaids.org/en/resources/909090.
-
- Direção Geral da Saúde, Instituto Nacional de Saúde Doutor Ricardo Jorge . Infeção VIH e SIDA Em Portugal-2019. Direção Geral de Saúde (DGS); Lisbon, Portugal: 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

