How the Pathological Microenvironment Affects the Behavior of Mesenchymal Stem Cells in the Idiopathic Pulmonary Fibrosis
- PMID: 33143370
- PMCID: PMC7662966
- DOI: 10.3390/ijms21218140
How the Pathological Microenvironment Affects the Behavior of Mesenchymal Stem Cells in the Idiopathic Pulmonary Fibrosis
Abstract
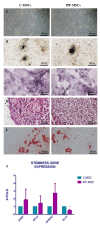
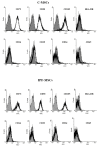
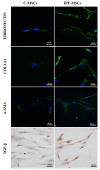
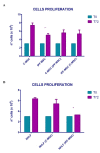
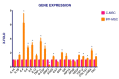
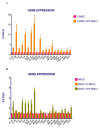
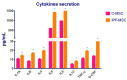
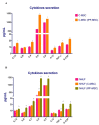
Idiopathic pulmonary fibrosis (IPF) is a chronic disease characterized by fibroblasts activation, ECM accumulation, and diffused alveolar inflammation. The role of inflammation in IPF is still controversial and its involvement may follow nontraditional mechanisms. It is seen that a pathological microenvironment may affect cells, in particular mesenchymal stem cells (MSCs) that may be able to sustain the inflamed microenvironment and influence the surrounding cells. Here MSCs have been isolated from fibrotic (IPF-MSCs) and control (C-MSCs) lung tissue; first cells were characterized and compared by the expression of molecules related to ECM, inflammation, and other interdependent pathways such as hypoxia and oxidative stress. Subsequently, MSCs were co-cultured between them and with NHLF to test the effects of the cellular crosstalk. Results showed that pathological microenvironment modified the features of MSCs: IPF-MSCs, compared to C-MSCs, express higher level of molecules related to ECM, inflammation, oxidative stress, and hypoxia; notably, when co-cultured with C-MSCs and NHLF, IPF-MSCs are able to induce a pathological phenotype on the surrounding cell types. In conclusion, in IPF the pathological microenvironment affects MSCs that in turn can modulate the behavior of other cell types favoring the progression of IPF.
Keywords: idiopathic pulmonary fibrosis; inflammation; mesenchymal stem cells; paracrine effect.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Padilla M. Idiopathic pulmonary fibrosis: The role of pathobiology in making a definitive diagnosis. Am. J. Manag. Care. 2015;21:s276–s283. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

