Age-Dependent and Sleep/Seizure-Induced Pathomechanisms of Autosomal Dominant Sleep-Related Hypermotor Epilepsy
- PMID: 33143372
- PMCID: PMC7662760
- DOI: 10.3390/ijms21218142
Age-Dependent and Sleep/Seizure-Induced Pathomechanisms of Autosomal Dominant Sleep-Related Hypermotor Epilepsy
Abstract
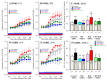
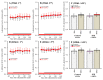
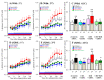
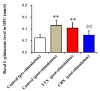
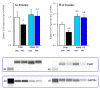
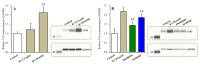
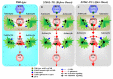
The loss-of-function S284L-mutant α4 subunit of the nicotinic acetylcholine receptor (nAChR) is considered to contribute to the pathomechanism of autosomal dominant sleep-related hypermotor epilepsy (ADSHE); however, the age-dependent and sleep-related pathomechanisms of ADSHE remain to be clarified. To explore the age-dependent and sleep-induced pathomechanism of ADSHE, the present study determined the glutamatergic transmission abnormalities associated with α4β2-nAChR and the astroglial hemichannel in the hyperdirect and corticostriatal pathways of ADSHE model transgenic rats (S286L-TG) bearing the rat S286L-mutant Chrna4 gene corresponding to the human S284L-mutant CHRNA4 gene of ADSHE, using multiprobe microdialysis and capillary immunoblotting analyses. This study could not detect glutamatergic transmission in the corticostriatal pathway from the orbitofrontal cortex (OFC) to the striatum. Before ADSHE onset (four weeks of age), functional abnormalities of glutamatergic transmission compared to the wild-type in the cortical hyperdirect pathway, from OFC to the subthalamic nucleus (STN) in S286L-TG, could not be detected. Conversely, after ADSHE onset (eight weeks of age), glutamatergic transmission in the hyperdirect pathway of S286L-TG was enhanced compared to the wild-type. Notably, enhanced glutamatergic transmission of S286L-TG was revealed by hemichannel activation in the OFC. Expression of connexin43 (Cx43) in the OFC of S286L-TG was upregulated after ADSHE onset but was almost equal to the wild-type prior to ADSHE onset. Differences in the expression of phosphorylated protein kinase B (pAkt) before ADSHE onset between the wild-type and S286L-TG were not observed; however, after ADSHE onset, pAkt was upregulated in S286L-TG. Conversely, the expression of phosphorylated extracellular signal-regulated kinase (pErk) was already upregulated before ADSHE onset compared to the wild-type. Both before and after ADSHE onset, subchronic nicotine administration decreased and did not affect the both expression of Cx43 and pErk of respective wild-type and S286L-TG, whereas the pAkt expression of both the wild-type and S286L-TG was increased by nicotine. Cx43 expression in the plasma membrane of the primary cultured astrocytes of the wild-type was increased by elevation of the extracellular K+ level (higher than 10 mM), and the increase in Cx43 expression in the plasma membrane required pErk functions. These observations indicate that a combination of functional abnormalities, GABAergic disinhibition, and upregulated pErk induced by the loss-of-function S286L-mutant α4β2-nAChR contribute to the age-dependent and sleep-induced pathomechanism of ADSHE via the upregulation/hyperactivation of the Cx43 hemichannels.
Keywords: L-glutamate; autosomal dominant sleep-related hypermotor epilepsy; basal ganglia; extracellular signal-regulated kinase; hemichannel; protein kinase B.
Conflict of interest statement
The authors state no conflict of interest.
Figures

References
-
- Okada M., Zhu G., Yoshida S., Kaneko S. Validation criteria for genetic animal models of epilepsy. Epilepsy Seizure. 2010;3:109–120. doi: 10.3805/eands.3.109. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

