Lipopeptide Epimers and a Phthalide Glycerol Ether with AChE Inhibitory Activities from the Marine-Derived Fungus Cochliobolus Lunatus SCSIO41401
- PMID: 33143384
- PMCID: PMC7693918
- DOI: 10.3390/md18110547
Lipopeptide Epimers and a Phthalide Glycerol Ether with AChE Inhibitory Activities from the Marine-Derived Fungus Cochliobolus Lunatus SCSIO41401
Abstract
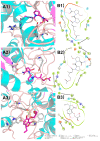
A pair of novel lipopeptide epimers, sinulariapeptides A (1) and B (2), and a new phthalide glycerol ether (3) were isolated from the marine algal-associated fungus Cochliobolus lunatus SCSIO41401, together with three known chromanone derivates (4-6). The structures of the new compounds, including the absolute configurations, were determined by comprehensive spectroscopic methods, experimental and calculated electronic circular dichroism (ECD), and Mo2 (OAc)4-induced ECD methods. The new compounds 1-3 showed moderate inhibitory activity against acetylcholinesterase (AChE), with IC50 values of 1.3-2.5 μM, and an in silico molecular docking study was also performed.
Keywords: AChE inhibitory; Cochliobolus lunatus; absolute configurations; lipopeptides; phthalide.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



Similar articles
-
Cytotoxic and Antibacterial Eremophilane Sesquiterpenes from the Marine-Derived Fungus Cochliobolus lunatus SCSIO41401.J Nat Prod. 2018 Jun 22;81(6):1405-1410. doi: 10.1021/acs.jnatprod.8b00015. Epub 2018 May 22. J Nat Prod. 2018. PMID: 29786436
-
Bisabolanoic acid A, a new polychiral sesquiterpene with AChE inhibitory activity from a mangrove-derived fungus Colletotrichum sp.J Asian Nat Prod Res. 2022 Jan;24(1):88-95. doi: 10.1080/10286020.2021.1873297. Epub 2021 Feb 3. J Asian Nat Prod Res. 2022. PMID: 33533666
-
Benzofurans from Styrax agrestis as acetylcholinesterase inhibitors: structure-activity relationships and molecular modeling studies.J Nat Prod. 2011 Oct 28;74(10):2081-8. doi: 10.1021/np200308j. Epub 2011 Sep 22. J Nat Prod. 2011. PMID: 21939219
-
Discovery of Enzyme Inhibitors from Mangrove Sediment Derived Fungus Trichoderma harzianum SCSIO 41051.Chem Biodivers. 2024 Apr;21(4):e202400070. doi: 10.1002/cbdv.202400070. Epub 2024 Mar 6. Chem Biodivers. 2024. PMID: 38356321
-
New phthalide derivatives from the Biscogniauxia sp. and their activities.Fitoterapia. 2019 Sep;137:104184. doi: 10.1016/j.fitote.2019.104184. Epub 2019 May 28. Fitoterapia. 2019. PMID: 31145983
Cited by
-
Isolation and acetylcholinesterase inhibitory activity of asterric acid derivatives produced by Talaromyces aurantiacus FL15, an endophytic fungus from Huperzia serrata.3 Biotech. 2022 Mar;12(3):60. doi: 10.1007/s13205-022-03125-2. Epub 2022 Feb 5. 3 Biotech. 2022. PMID: 35186657 Free PMC article.
-
Cyclic Peptides from the Soft Coral-Derived Fungus Aspergillus sclerotiorum SCSIO 41031.Mar Drugs. 2021 Dec 10;19(12):701. doi: 10.3390/md19120701. Mar Drugs. 2021. PMID: 34940700 Free PMC article.
-
Diversified Chaetoglobosins from the Marine-Derived Fungus Emericellopsis sp. SCSIO41202.Molecules. 2022 Mar 11;27(6):1823. doi: 10.3390/molecules27061823. Molecules. 2022. PMID: 35335187 Free PMC article.
-
DPPH Radical Scavenging Activity of New Phenolics from the Fermentation Broth of Mushroom Morehella importuna.Molecules. 2023 Jun 14;28(12):4760. doi: 10.3390/molecules28124760. Molecules. 2023. PMID: 37375314 Free PMC article.
-
Microorganism-Derived Molecules as Enzyme Inhibitors to Target Alzheimer's Diseases Pathways.Pharmaceuticals (Basel). 2023 Apr 12;16(4):580. doi: 10.3390/ph16040580. Pharmaceuticals (Basel). 2023. PMID: 37111337 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- 81973235, 21977102/National Natural Science Foundation of China
- 2019B151502042/Guangdong Basic and Applied Basic Research Foundation
- 2020B1111030005/Key-Area Research and Development Program of Guangdong Province
- [2019]A28, [2020]033, [2020]037, [2020]039/Special Funds for Promoting Economic Development (Marine Economic Development) of Guangdong Province
- 2019BT02Y262/Guangdong Local Innovation Team Program
LinkOut - more resources
Full Text Sources
Other Literature Sources

