Dialkyl Ether Formation at High-Valent Nickel
- PMID: 33143423
- PMCID: PMC7677934
- DOI: 10.1021/jacs.0c07381
Dialkyl Ether Formation at High-Valent Nickel
Abstract
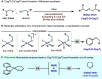
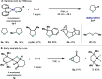
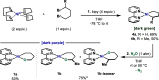
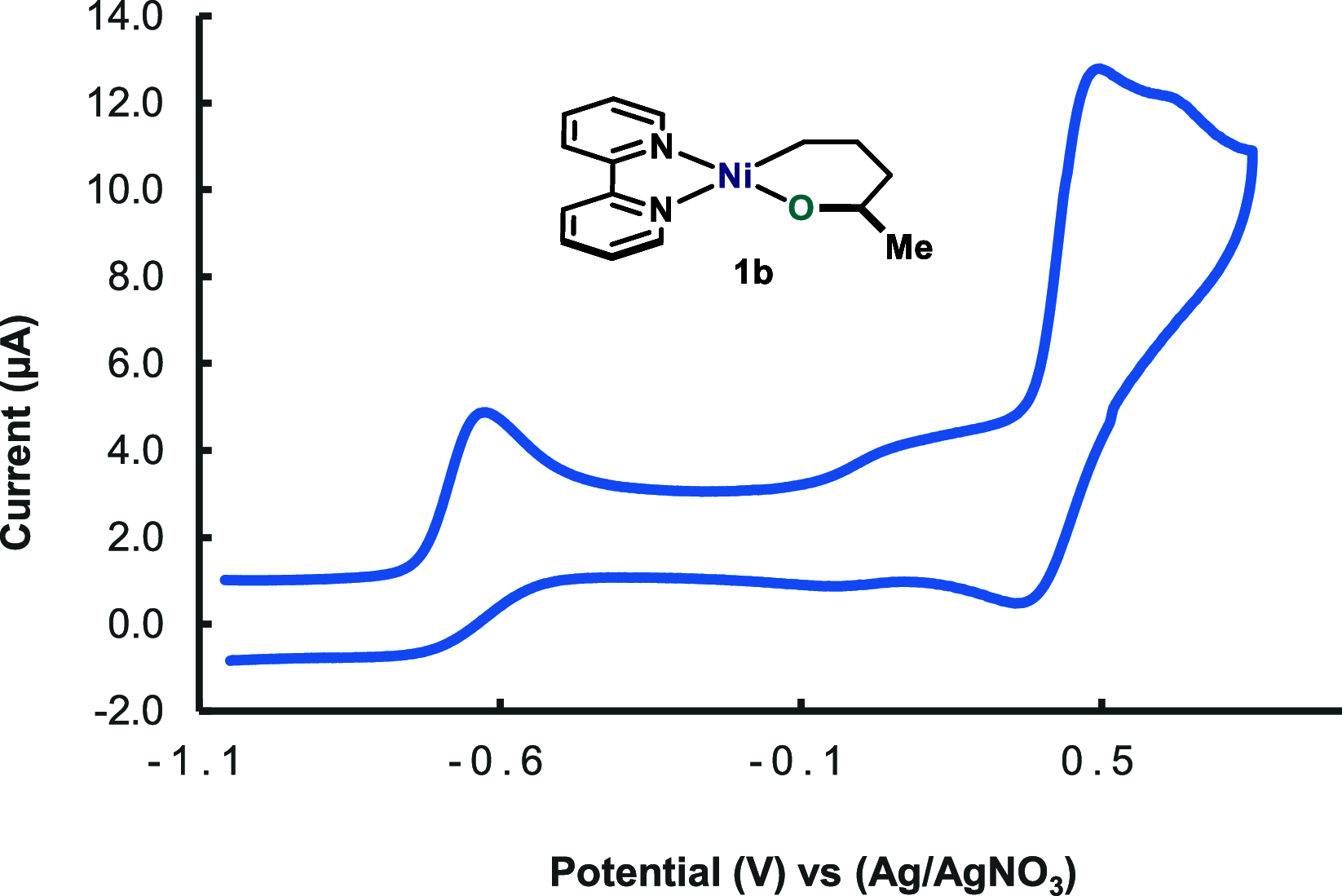
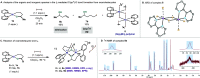
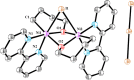
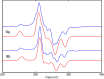
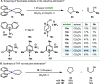
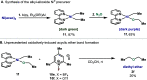
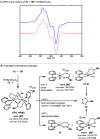
In this article, we investigated the I2-promoted cyclic dialkyl ether formation from 6-membered oxanickelacycles originally reported by Hillhouse. A detailed mechanistic investigation based on spectroscopic and crystallographic analysis revealed that a putative reductive elimination to forge C(sp3)-OC(sp3) using I2 might not be operative. We isolated a paramagnetic bimetallic NiIII intermediate featuring a unique Ni2(OR)2 (OR = alkoxide) diamond-like core complemented by a μ-iodo bridge between the two Ni centers, which remains stable at low temperatures, thus permitting its characterization by NMR, EPR, X-ray, and HRMS. At higher temperatures (>-10 °C), such bimetallic intermediate thermally decomposes to afford large amounts of elimination products together with iodoalkanols. Observation of the latter suggests that a C(sp3)-I bond reductive elimination occurs preferentially to any other challenging C-O bond reductive elimination. Formation of cyclized THF rings is then believed to occur through cyclization of an alcohol/alkoxide to the recently forged C(sp3)-I bond. The results of this article indicate that the use of F+ oxidants permits the challenging C(sp3)-OC(sp3) bond formation at a high-valent nickel center to proceed in good yields while minimizing deleterious elimination reactions. Preliminary investigations suggest the involvement of a high-valent bimetallic NiIII intermediate which rapidly extrudes the C-O bond product at remarkably low temperatures. The new set of conditions permitted the elusive synthesis of diethyl ether through reductive elimination, a remarkable feature currently beyond the scope of Ni.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
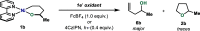
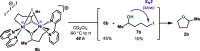
Similar articles
-
Identifying the Imperative Role of Metal-Olefin Interactions in Catalytic C-O Reductive Elimination from Nickel(II).ACS Catal. 2021 Aug 20;11(16):10208-10222. doi: 10.1021/acscatal.1c02790. Epub 2021 Aug 2. ACS Catal. 2021. PMID: 35186424 Free PMC article.
-
Bimetallic C-C Bond-Forming Reductive Elimination from Nickel.J Am Chem Soc. 2016 Apr 13;138(14):4779-86. doi: 10.1021/jacs.6b00016. Epub 2016 Apr 4. J Am Chem Soc. 2016. PMID: 27005998
-
N-N Bond Forming Reductive Elimination via a Mixed-Valent Nickel(II)-Nickel(III) Intermediate.Angew Chem Int Ed Engl. 2016 Jun 20;55(26):7534-8. doi: 10.1002/anie.201602566. Epub 2016 May 4. Angew Chem Int Ed Engl. 2016. PMID: 27144682
-
Binuclear, High-Valent Nickel Complexes: Ni-Ni Bonds in Aryl-Halogen Bond Formation.Angew Chem Int Ed Engl. 2017 Mar 20;56(13):3635-3639. doi: 10.1002/anie.201611572. Epub 2017 Feb 14. Angew Chem Int Ed Engl. 2017. PMID: 28194838
-
Electrophilic halogenation-reductive elimination chemistry of organopalladium and -platinum complexes.Acc Chem Res. 2015 Feb 17;48(2):238-47. doi: 10.1021/ar500325x. Epub 2015 Jan 20. Acc Chem Res. 2015. PMID: 25602260 Review.
Cited by
-
Mediator-Enabled Electrocatalysis with Ligandless Copper for Anaerobic Chan-Lam Coupling Reactions.J Am Chem Soc. 2021 Apr 28;143(16):6257-6265. doi: 10.1021/jacs.1c02103. Epub 2021 Apr 16. J Am Chem Soc. 2021. PMID: 33861580 Free PMC article.
-
Direct synthesis of ethers from alcohols & aldehydes enabled by an oxocarbenium ion interception strategy.Chem Sci. 2025 Mar 4;16(16):6991-7003. doi: 10.1039/d4sc06203e. eCollection 2025 Apr 16. Chem Sci. 2025. PMID: 40134658 Free PMC article.
-
Mechanism of Ni-Catalyzed Photochemical Halogen Atom-Mediated C(sp3)-H Arylation.J Am Chem Soc. 2024 Jun 5;146(22):15331-15344. doi: 10.1021/jacs.4c03099. Epub 2024 May 22. J Am Chem Soc. 2024. PMID: 38778454 Free PMC article.
-
Carbon-centered radical capture at nickel(II) complexes: Spectroscopic evidence, rates, and selectivity.Chem. 2023 May 11;9(5):1295-1308. doi: 10.1016/j.chempr.2023.02.010. Epub 2023 Mar 13. Chem. 2023. PMID: 40786745 Free PMC article.
-
Ligand-field transition-induced C-S bond formation from nickelacycles.Chem Sci. 2021 Nov 10;12(48):15908-15915. doi: 10.1039/d1sc05113j. eCollection 2021 Dec 15. Chem Sci. 2021. PMID: 35024114 Free PMC article.
References
-
- Roughley S. D.; Jordan A. M. The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 2011, 54, 3451.10.1021/jm200187y. - DOI - PubMed
- Brown A.; Brown L.; Brown T. B.; Calabrese A.; Ellis D.; Puhalo N.; Smith C. R.; Wallace O.; Watson L. Triazole oxytocin antagonists: identification of aryl ether replacements for a biaryl substituent. Bioorg. Med. Chem. Lett. 2008, 18, 5242.10.1016/j.bmcl.2008.08.066. - DOI - PubMed
- Backes B. J.; Ellman J. A. Solid support linker strategies. Curr. Opin. Chem. Biol. 1997, 1, 86.10.1016/S1367-5931(97)80113-5. - DOI - PubMed
- Ganesan A.Wang resin. Encyclopedia of reagents for organic synthesis; Wiley: Hoboken, NJ, 2003.
- Harris J. M.Poly(ethylene glycol) Chemistry: Biotechnical and Biomedical Applications; Plenum Press: New York, 1992.
-
- Williamson A. W. J. Chem. Soc. 1852, 4, 229.10.1039/QJ8520400229. - DOI
-
Alexander William Williamson (1824–1904) discovered this reaction in 1850 at University College, London
-
-
For selected reviews on Williamson ether synthesis, see:
- Li J. J.Williamson ether synthesis. Name Reactions; Springer:Cham, 2014.
- Wang Z.Williamson Ether Synthesis. Comprehensive Organic Name Reactions and Reagents; John Wiley & Sons: Hoboken, NJ, 2010; pp 3026–3030.
-
-
- Xiang J.; Shang M.; Kawamata Y.; Lundberg H.; Reisberg S.; Chen M.; Mykhailiuk P.; Beutner G.; Collins M.; Davies A.; Del Bel M.; Gallego G.; Spangler J.; Starr J. T.; Yang S.; Blackmond D.; Baran P. S. Hindered Dialkyl Ether Synthesis via Electrogenerated Carbocations. Nature 2019, 573, 398.10.1038/s41586-019-1539-y. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

