Dialkyl Ether Formation at High-Valent Nickel
- PMID: 33143423
- PMCID: PMC7677934
- DOI: 10.1021/jacs.0c07381
Dialkyl Ether Formation at High-Valent Nickel
Abstract
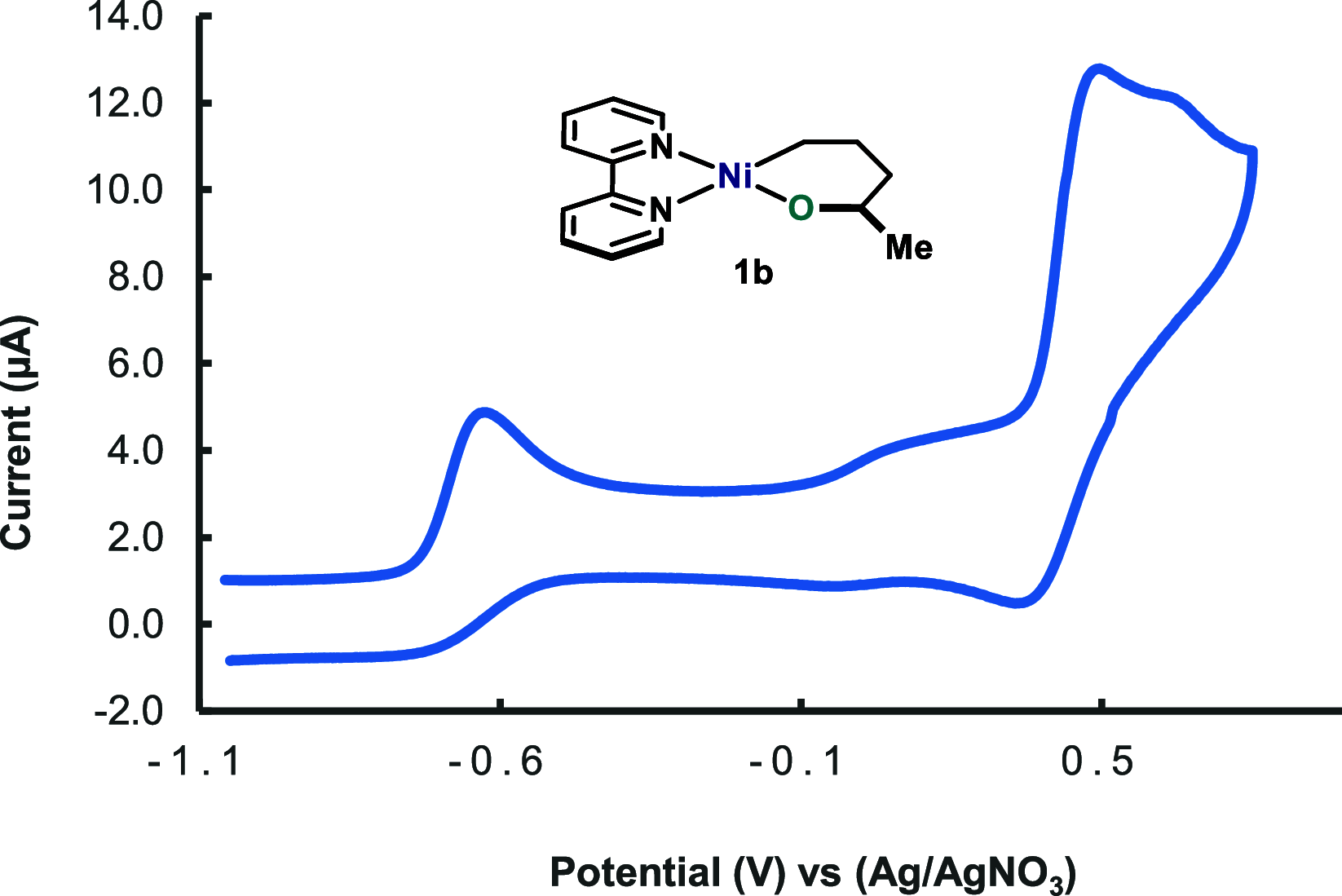
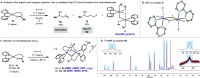
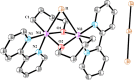
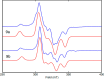
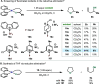
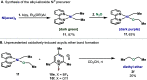
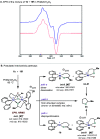
In this article, we investigated the I2-promoted cyclic dialkyl ether formation from 6-membered oxanickelacycles originally reported by Hillhouse. A detailed mechanistic investigation based on spectroscopic and crystallographic analysis revealed that a putative reductive elimination to forge C(sp3)-OC(sp3) using I2 might not be operative. We isolated a paramagnetic bimetallic NiIII intermediate featuring a unique Ni2(OR)2 (OR = alkoxide) diamond-like core complemented by a μ-iodo bridge between the two Ni centers, which remains stable at low temperatures, thus permitting its characterization by NMR, EPR, X-ray, and HRMS. At higher temperatures (>-10 °C), such bimetallic intermediate thermally decomposes to afford large amounts of elimination products together with iodoalkanols. Observation of the latter suggests that a C(sp3)-I bond reductive elimination occurs preferentially to any other challenging C-O bond reductive elimination. Formation of cyclized THF rings is then believed to occur through cyclization of an alcohol/alkoxide to the recently forged C(sp3)-I bond. The results of this article indicate that the use of F+ oxidants permits the challenging C(sp3)-OC(sp3) bond formation at a high-valent nickel center to proceed in good yields while minimizing deleterious elimination reactions. Preliminary investigations suggest the involvement of a high-valent bimetallic NiIII intermediate which rapidly extrudes the C-O bond product at remarkably low temperatures. The new set of conditions permitted the elusive synthesis of diethyl ether through reductive elimination, a remarkable feature currently beyond the scope of Ni.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
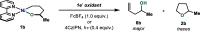
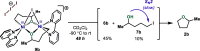
References
-
- Roughley S. D.; Jordan A. M. The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 2011, 54, 3451.10.1021/jm200187y. - DOI - PubMed
- Brown A.; Brown L.; Brown T. B.; Calabrese A.; Ellis D.; Puhalo N.; Smith C. R.; Wallace O.; Watson L. Triazole oxytocin antagonists: identification of aryl ether replacements for a biaryl substituent. Bioorg. Med. Chem. Lett. 2008, 18, 5242.10.1016/j.bmcl.2008.08.066. - DOI - PubMed
- Backes B. J.; Ellman J. A. Solid support linker strategies. Curr. Opin. Chem. Biol. 1997, 1, 86.10.1016/S1367-5931(97)80113-5. - DOI - PubMed
- Ganesan A.Wang resin. Encyclopedia of reagents for organic synthesis; Wiley: Hoboken, NJ, 2003.
- Harris J. M.Poly(ethylene glycol) Chemistry: Biotechnical and Biomedical Applications; Plenum Press: New York, 1992.
-
- Williamson A. W. J. Chem. Soc. 1852, 4, 229.10.1039/QJ8520400229. - DOI
-
Alexander William Williamson (1824–1904) discovered this reaction in 1850 at University College, London
-
-
For selected reviews on Williamson ether synthesis, see:
- Li J. J.Williamson ether synthesis. Name Reactions; Springer:Cham, 2014.
- Wang Z.Williamson Ether Synthesis. Comprehensive Organic Name Reactions and Reagents; John Wiley & Sons: Hoboken, NJ, 2010; pp 3026–3030.
-
-
- Xiang J.; Shang M.; Kawamata Y.; Lundberg H.; Reisberg S.; Chen M.; Mykhailiuk P.; Beutner G.; Collins M.; Davies A.; Del Bel M.; Gallego G.; Spangler J.; Starr J. T.; Yang S.; Blackmond D.; Baran P. S. Hindered Dialkyl Ether Synthesis via Electrogenerated Carbocations. Nature 2019, 573, 398.10.1038/s41586-019-1539-y. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

