Desialylation of platelet surface glycans enhances platelet adhesion to adsorbent polymers for lipoprotein apheresis
- PMID: 33143534
- PMCID: PMC8524686
- DOI: 10.1177/0391398820968849
Desialylation of platelet surface glycans enhances platelet adhesion to adsorbent polymers for lipoprotein apheresis
Abstract
Background: Lipoprotein apheresis is an important therapeutic option in homozygous familial hypercholesterolemia, progressive atherosclerosis, or when depletion of lipoprotein(a) is indicated. It is generally regarded as safe, but drops in platelet counts as well as sporadic episodes of thrombocytopenia have been reported. We assessed the influence of platelet desialylation, which may be induced by endogenous or pathogen-derived neuraminidases, on platelet adhesion to polyacrylate-based adsorbents for whole blood lipoprotein apheresis.
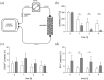
Methods: Medical grade platelet concentrates were incubated with neuraminidase in vitro and were circulated over adsorbent columns downscaled from clinical application.
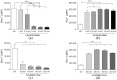
Results: Cleavage of terminal sialic residues resulted in platelet activation with significantly elevated expression of platelet factor 4 (PF4) and in enhanced platelet adhesion to the adsorbent, accompanied by a pronounced drop in platelet counts in the column flow-through.
Conclusion: Alterations in endogenous neuraminidase activity or exogenous (pathogen-derived) neuraminidase may trigger enhanced platelet adhesion in whole blood lipoprotein apheresis.
Keywords: Platelets; adsorption; glycosylation; sialylation.
Conflict of interest statement
Figures

References
-
- Saeed A, Virani SS. Lipoprotein(a) and cardiovascular disease: current state and future directions for an enigmatic lipoprotein. Front Biosci (Landmark Ed.) 2018; 23: 1099–1112. - PubMed
-
- Santos RD, Gidding SS, Hegele RA, et al. Defining severe familial hypercholesterolaemia and the implications for clinical management: a consensus statement from the international atherosclerosis society severe familial hypercholesterolemia panel. Lancet Diabetes Endocrinol 2016; 4: 850–861. - PubMed
-
- Stefanutti C, Julius U, Watts GF, et al. Toward an international consensus—integrating lipoprotein apheresis and new lipid-lowering drugs. J Clin Lipidol 2017; 11: 858–871, e3. - PubMed
-
- Alonso R, Andres E, Mata N, et al. Lipoprotein(a) levels in familial hypercholesterolemia: an important predictor of cardiovascular disease independent of the type of LDL receptor mutation. J Am Coll Cardiol 2014; 63: 1982–1989. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

