E3 Ligase Ligands for PROTACs: How They Were Found and How to Discover New Ones
- PMID: 33143537
- PMCID: PMC8013866
- DOI: 10.1177/2472555220965528
E3 Ligase Ligands for PROTACs: How They Were Found and How to Discover New Ones
Abstract
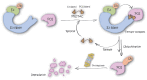
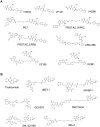
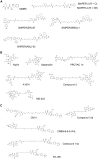
Bifunctional degrader molecules, also called proteolysis-targeting chimeras (PROTACs), are a new modality of chemical tools and potential therapeutics to understand and treat human disease. A required PROTAC component is a ligand binding to an E3 ubiquitin ligase, which is then joined to another ligand binding to a protein to be degraded via the ubiquitin-proteasome system. The advent of nonpeptidic small-molecule E3 ligase ligands, notably for von Hippel-Lindau (VHL) and cereblon (CRBN), revolutionized the field and ushered in the design of drug-like PROTACs with potent and selective degradation activity. A first wave of PROTAC drugs are now undergoing clinical development in cancer, and the field is seeking to extend the repertoire of chemistries that allow hijacking new E3 ligases to improve the scope of targeted protein degradation.Here, we briefly review how traditional E3 ligase ligands were discovered, and then outline approaches and ligands that have been recently used to discover new E3 ligases for PROTACs. We will then take an outlook at current and future strategies undertaken that invoke either target-based screening or phenotypic-based approaches, including the use of DNA-encoded libraries (DELs), display technologies and cyclic peptides, smaller molecular glue degraders, and covalent warhead ligands. These approaches are ripe for expanding the chemical space of PROTACs and usher in the advent of other emerging bifunctional modalities of proximity-based pharmacology.
Keywords: E3 ubiquitin ligase; PROTACs; binding ligands; molecular glues; targeted protein degradation.
Conflict of interest statement
Figures



References
-
- Schneekloth J. S., Jr., Fonseca F. N., Koldobskiy M; et al. Chemical Genetic Control of Protein Levels: Selective In Vivo Targeted Degradation. J. Am. Chem. Soc. 2004, 126, 3748–3754. - PubMed
-
- Itoh Y., Ishikawa M., Naito M.; et al. Protein Knockdown Using Methyl Bestatin-Ligand Hybrid Molecules: Design and Synthesis of Inducers of Ubiquitination-Mediated Degradation of Cellular Retinoic Acid-Binding Proteins. J. Am. Chem. Soc. 2010, 132, 5820–5826. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

