Randomized, placebo-controlled, double-blind, pilot trial to investigate safety and efficacy of Cerebrolysin in patients with aneurysmal subarachnoid hemorrhage
- PMID: 33143640
- PMCID: PMC7607674
- DOI: 10.1186/s12883-020-01908-9
Randomized, placebo-controlled, double-blind, pilot trial to investigate safety and efficacy of Cerebrolysin in patients with aneurysmal subarachnoid hemorrhage
Abstract
Asbtract: BACKGROUND: There are limited neuroprotective treatment options for patients with aneurysmal subarachnoid hemorrhage (SAH). Cerebrolysin, a brain-specific proposed pleiotropic neuroprotective agent, has been suggested to improve global functional outcomes in ischemic stroke. We investigated the efficacy, safety and feasibility of administering Cerebrolysin for SAH patients.
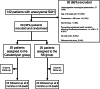
Methods: This was a prospective, randomized, double-blind, placebo-controlled, single-center, parallel-group pilot study. Fifty patients received either daily Cerebrolysin (30 ml/day) or a placebo (saline) for 14 days (25 patients per study group). The primary endpoint was a favorable Extended Glasgow Outcome Scale (GOSE) of 5 to 8 (moderate disability to good recovery) at six-months. Secondary endpoints included the modified Ranking Scale (mRS), the Montreal Cognitive Assessment (MOCA) score, occurrence of adverse effects and the occurrence of delayed cerebral ischemia (DCI).
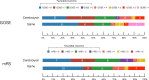
Results: No severe adverse effects or mortality attributable to Cerebrolysin were observed. No significant difference was detected in the proportion of patients with favorable six-month GOSE in either study group (odds ratio (OR): 1.49; 95% confidence interval (CI): 0.43-5.17). Secondary functional outcome measures for favorable six-month recovery i.e. a mRS of 0 to 3 (OR: 3.45; 95% CI 0.79-15.01) were comparable for both groups. Similarly, there was no difference in MOCA neurocognitive performance (p-value: 0.75) and in the incidence of DCI (OR: 0.85 95% CI: 0.28-2.59).
Conclusions: Use of Cerebrolysin in addition to standard-of-care management of aneurysmal SAH is safe, well tolerated and feasible. However, the neutral results of this trial suggest that it does not improve the six-month global functional performance of patients.
Clinical trial registration: Name of Registry: ClinicalTrials.gov Trial Registration Number: NCT01787123 . Date of Registration: 8th February 2013.
Keywords: Aneurysmal subarachnoid hemorrhage; Delayed cerebral ischemia; Neuroprotection.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Cerebrolysin in patients with acute ischemic stroke in Asia: results of a double-blind, placebo-controlled randomized trial.Stroke. 2012 Mar;43(3):630-6. doi: 10.1161/STROKEAHA.111.628537. Epub 2012 Jan 26. Stroke. 2012. PMID: 22282884 Clinical Trial.
-
Neuroprotective effect of dapsone in patients with aneurysmal subarachnoid hemorrhage: a prospective, randomized, double-blind, placebo-controlled clinical trial.Neurosurg Focus. 2022 Mar;52(3):E12. doi: 10.3171/2021.12.FOCUS21663. Neurosurg Focus. 2022. PMID: 35231887 Clinical Trial.
-
Efficacy and safety of Cerebrolysin treatment in early recovery after acute ischemic stroke: a randomized, placebo-controlled, double-blinded, multicenter clinical trial.J Med Life. 2017 Jul-Sep;10(3):153-160. J Med Life. 2017. PMID: 29075343 Free PMC article. Clinical Trial.
-
Effect of statin treatment on vasospasm-related morbidity and functional outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis.J Neurosurg. 2017 Aug;127(2):291-301. doi: 10.3171/2016.5.JNS152900. Epub 2016 Oct 7. J Neurosurg. 2017. PMID: 27715439
-
Efficacy and Safety of Cerebrolysin for Acute Ischemic Stroke: A Meta-Analysis of Randomized Controlled Trials.Biomed Res Int. 2017;2017:4191670. doi: 10.1155/2017/4191670. Epub 2017 Jun 5. Biomed Res Int. 2017. PMID: 28656143 Free PMC article. Review.
Cited by
-
A systematic-search-and-review of registered pharmacological therapies investigated to improve neuro-recovery after a stroke.Front Neurol. 2024 Jan 31;15:1346177. doi: 10.3389/fneur.2024.1346177. eCollection 2024. Front Neurol. 2024. PMID: 38356890 Free PMC article.
-
Cerebrolysin in Patients with Subarachnoid Hemorrhage: A Systematic Review and Meta-Analysis.J Clin Med. 2023 Oct 20;12(20):6638. doi: 10.3390/jcm12206638. J Clin Med. 2023. PMID: 37892776 Free PMC article. Review.
-
Role of Cerebrolysin® in Rehabilitation in Ischemic Stroke: A Case Report.Am J Case Rep. 2021 Sep 8;22:e932365. doi: 10.12659/AJCR.932365. Am J Case Rep. 2021. PMID: 34493699 Free PMC article.
-
Outcomes Measures in Subarachnoid Hemorrhage Research.Transl Stroke Res. 2025 Feb;16(1):25-36. doi: 10.1007/s12975-024-01284-3. Epub 2024 Jul 29. Transl Stroke Res. 2025. PMID: 39073651 Review.
-
Systemic inflammatory markers of persistent cerebral edema after aneurysmal subarachnoid hemorrhage.J Neuroinflammation. 2022 Aug 4;19(1):199. doi: 10.1186/s12974-022-02564-1. J Neuroinflammation. 2022. PMID: 35927663 Free PMC article.
References
-
- Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366(9488):809–817. doi: 10.1016/S0140-6736(05)67214-5. - DOI - PubMed
-
- Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41(10):2391–2395. doi: 10.1161/STROKEAHA.110.589275. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

