Cellular infiltration in traumatic brain injury
- PMID: 33143727
- PMCID: PMC7640704
- DOI: 10.1186/s12974-020-02005-x
Cellular infiltration in traumatic brain injury
Abstract
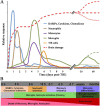
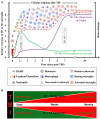
Traumatic brain injury leads to cellular damage which in turn results in the rapid release of damage-associated molecular patterns (DAMPs) that prompt resident cells to release cytokines and chemokines. These in turn rapidly recruit neutrophils, which assist in limiting the spread of injury and removing cellular debris. Microglia continuously survey the CNS (central nervous system) compartment and identify structural abnormalities in neurons contributing to the response. After some days, when neutrophil numbers start to decline, activated microglia and astrocytes assemble at the injury site-segregating injured tissue from healthy tissue and facilitating restorative processes. Monocytes infiltrate the injury site to produce chemokines that recruit astrocytes which successively extend their processes towards monocytes during the recovery phase. In this fashion, monocytes infiltration serves to help repair the injured brain. Neurons and astrocytes also moderate brain inflammation via downregulation of cytotoxic inflammation. Depending on the severity of the brain injury, T and B cells can also be recruited to the brain pathology sites at later time points.
Keywords: Cellular infiltration; Microglial dynamics; Neuroinflammation; Traumatic brain injury.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Finnie JW, Blumbergs PC. Traumatic brain injury. Vet Pathol. 2002;39:679–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

