Efficacy of Tong-Xie-Yao-Fang granule and its impact on whole transcriptome profiling in diarrhea-predominant irritable bowel syndrome patients: study protocol for a randomized controlled trial
- PMID: 33143731
- PMCID: PMC7607547
- DOI: 10.1186/s13063-020-04833-x
Efficacy of Tong-Xie-Yao-Fang granule and its impact on whole transcriptome profiling in diarrhea-predominant irritable bowel syndrome patients: study protocol for a randomized controlled trial
Abstract
Background: Irritable bowel syndrome (IBS) is one kind of common functional bowel disease with obscure pathogenesis, and exploration about whole transcriptome profiling in IBS-D is still negligible. Conventional medications have limited effects, which makes focus shifted to traditional Chinese medicine (TCM). Tong-Xie-Yao-Fang, as a classic herbal formula in TCM, is pretty effective and safe for the treatment of diarrhea-predominant irritable bowel syndrome (IBS-D), but the underlying therapeutic mechanism remains unknown. We aim to verify the efficacy and safety of TXYF granule (the formula particles mixed together) in IBS-D and elucidate the gene-level mechanism of IBS-D and therapeutic targets of TXYF granule based on whole transcriptome analysis.
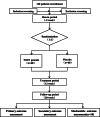
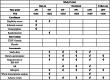
Methods/design: This is a randomized, double-blind, and placebo-controlled clinical trial consisting of 2 weeks of run-in period, 12 weeks of treatment period, and 8 weeks of follow-up period. We will enroll 120 participants with IBS-D, who will be randomly assigned to the TXYF granule group and the placebo group, and recruit additional 10 healthy individuals as controls for mechanistic outcome. The two groups respectively take TXYF granule or placebo orally for treatment. The primary outcome is the response rate of IBS-Symptom Severity Score (IBS-SSS). The secondary outcomes include adequate relief (AR), IBS-Quality of Life Questionnaire (IBS-QOL), and long-term efficacy. Mechanistic outcome is the whole transcriptome profiling of the intestinal mucosae from IBS participants before and after the treatment and healthy individuals.
Discussion: This trial will prove the effectiveness and safety of TXYF granule with high-quality evidence and provide a penetrating and comprehensive perspective on the molecular mechanism of IBS-D by whole transcriptome analysis, which makes us pinpoint specific biomarkers of IBS-D and therapeutic targets of TXYF.
Trial registration: Chinese Clinical Trial Registry ChiCTR-IOR-1900021785 . Registered on 9 March 2019.
Keywords: Diarrhea-predominant irritable bowel syndrome; Randomized controlled trial; Tong-Xie-Yao-Fang granule; Traditional Chinese medicine; Whole transcriptome profiling.
Conflict of interest statement
All authors declare no competing interests.
Figures
References
-
- Wouters MM, Balemans D, Van Wanrooy S, Dooley J, Cibert-Goton V, Alpizar YA, et al. Histamine receptor H1-mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology. 2016;150(4):875–87.e9. doi: 10.1053/j.gastro.2015.12.034. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 81804047/National Natural Science Foundation of China
- 81774264/National Natural Science Foundation of China
- -/key research projects of first-class disciplines of Guangzhou University of Chinese Medicine
- 2017TD05/Innovative Research Team Project of "Innovative Strong Institute", the First Affiliated Hospital of Guangzhou University of Traditional Chinese Medicine
- 20181095/Guangdong Provincial Traditional Chinese Medicine Research Project
LinkOut - more resources
Full Text Sources
Research Materials

