Misfolding, altered membrane distributions, and the unfolded protein response contribute to pathogenicity differences in Na,K-ATPase ATP1A3 mutations
- PMID: 33144327
- PMCID: PMC7949067
- DOI: 10.1074/jbc.RA120.015271
Misfolding, altered membrane distributions, and the unfolded protein response contribute to pathogenicity differences in Na,K-ATPase ATP1A3 mutations
Abstract
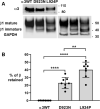
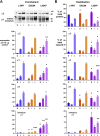
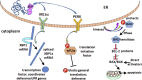
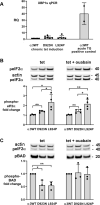
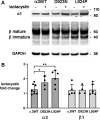
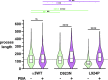
Missense mutations in ATP1A3, the α3 isoform of Na,K-ATPase, cause neurological phenotypes that differ greatly in symptoms and severity. A mechanistic basis for differences is lacking, but reduction of activity alone cannot explain them. Isogenic cell lines with endogenous α1 and inducible exogenous α3 were constructed to compare mutation properties. Na,K-ATPase is made in the endoplasmic reticulum (ER), but the glycan-free catalytic α subunit complexes with glycosylated β subunit in the ER to proceed through Golgi and post-Golgi trafficking. We previously observed classic evidence of protein misfolding in mutations with severe phenotypes: differences in ER retention of endogenous β1 subunit, impaired trafficking of α3, and cytopathology, suggesting that they misfold during biosynthesis. Here we tested two mutations associated with different phenotypes: D923N, which has a median age of onset of hypotonia or dystonia at 3 years, and L924P, with severe infantile epilepsy and profound impairment. Misfolding during biosynthesis in the ER activates the unfolded protein response, a multiarmed program that enhances protein folding capacity, and if that fails, triggers apoptosis. L924P showed more nascent protein retention in ER than D923N; more ER-associated degradation of α3 (ERAD); larger differences in Na,K-ATPase subunit distributions among subcellular fractions; and greater inactivation of eIF2α, a major defensive step of the unfolded protein response. In L924P there was also altered subcellular distribution of endogenous α1 subunit, analogous to a dominant negative effect. Both mutations showed pro-apoptotic sensitization by reduced phosphorylation of BAD. Encouragingly, however, 4-phenylbutyrate, a pharmacological corrector, reduced L924P ER retention, increased α3 expression, and restored morphology.
Keywords: 4-phenylbutyrate (4PBA); N-linked glycosylation; Na(+)/K(+)-ATPase; endoplasmic reticulum associated protein degradation (ERAD); endoplasmic reticulum stress (ER stress); eukaryotic initiation factor 2alpha (eIF2 α); genetic disease; protein misfolding; subcellular fractionation; unfolded protein response (UPR).
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Lassuthova P., Rebelo A.P., Ravenscroft G., Lamont P.J., Davis M.R., Manganelli F., Feely S.M., Bacon C., Brozkova D.S., Haberlova J., Mazanec R., Tao F., Saghira C., Abreu L., Courel S. Mutations in ATP1A1 cause dominant Charcot-Marie-Tooth type 2. Am. J. Hum. Genet. 2018;102:505–514. - PMC - PubMed
-
- Dard R., Mignot C., Durr A., Lesca G., Sanlaville D., Roze E., Mochel F. Relapsing encephalopathy with cerebellar ataxia related to an ATP1A3 mutation. Dev. Med. Child. Neurol. 2015;57:1183–1186. - PubMed
-
- Yano S.T., Silver K., Young R., DeBrosse S.D., Ebel R.S., Swoboda K.J., Acsadi G. Fever-induced paroxysmal weakness and encephalopathy, a new phenotype of ATP1A3 mutation. Pediatr. Neurol. 2017;73:101–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

