HY5 and phytochrome activity modulate shoot-to-root coordination during thermomorphogenesis in Arabidopsis
- PMID: 33144393
- PMCID: PMC7758624
- DOI: 10.1242/dev.192625
HY5 and phytochrome activity modulate shoot-to-root coordination during thermomorphogenesis in Arabidopsis
Abstract
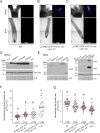
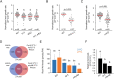
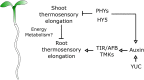
Temperature is one of the most impactful environmental factors to which plants adjust their growth and development. Although the regulation of temperature signaling has been extensively investigated for the aerial part of plants, much less is known and understood about how roots sense and modulate their growth in response to fluctuating temperatures. Here, we found that shoot and root growth responses to high ambient temperature are coordinated during early seedling development in Arabidopsis A shoot signaling module that includes HY5, the phytochromes and the PIFs exerts a central function in coupling these growth responses and maintaining auxin levels in the root. In addition to the HY5/PIF-dependent shoot module, a regulatory axis composed of auxin biosynthesis and auxin perception factors controls root responses to high ambient temperature. Taken together, our findings show that shoot and root developmental responses to temperature are tightly coupled during thermomorphogenesis and suggest that roots integrate energy signals with local hormonal inputs.
Keywords: Arabidopsis; HY5; Phytochromes; Root development; Temperature; Thermomorphogenesis.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

