Genomic Profiling and Clinicopathological Characteristics of Neuroendocrine Tumors of the Lung in East Asian Patients
- PMID: 33144445
- PMCID: PMC7811643
- DOI: 10.21873/invivo.12176
Genomic Profiling and Clinicopathological Characteristics of Neuroendocrine Tumors of the Lung in East Asian Patients
Abstract
Background/aim: In recent years, the genomic landscape of neuroendocrine tumors (NETs) of the lung has been investigated. However, more data are necessary to elucidate the heterogeneous nature of NETs, especially in East Asian patients.
Patients and methods: A total of 64 patients who underwent surgical resection for lung NETs [26 typical or atypical carcinoid tumors, 21 large-cell neuroendocrine carcinomas (LCNECs), and 19 small-cell lung carcinomas (SCLCs)] were enrolled, and samples from 46 patients were subjected to targeted next-generation sequencing.
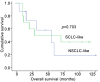
Results: Co-mutations of tumor protein p53 (TP53) and RB transcriptional corepressor 1 (RB1) were detected in 15%, 42%, and 93% of carcinoid tumors, LCNECs, and SCLCs, respectively. Oncogenic or targetable genetic alterations identified in this study included mutations of KRAS proto-oncogene (KRAS), phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), ALK receptor tyrosine kinase (ALK), mitogen-activated protein kinase kinase 1 (MAP2K1), and isocitrate dehydrogenase 1 (IDH1), as well as amplifications of erb-b2 receptor tyrosine kinase 2 (ERBB2), fibroblast growth factor receptor 1 (FGFR1), CD274 molecule (CD274), and MYCN proto-oncogene (MYCN). These alterations were more frequently found in high-grade NETs than in carcinoid tumors (33.3% vs. 7.7%). Programmed cell death-ligand 1 expression was strongly associated with the LCNEC subtype among NETs (p=0.002).
Conclusion: The mutational status of TP53 and RB1 was significantly associated with NET subtypes in East Asian patients. Targeted therapy or immunotherapy may serve as a treatment option in a subset of patients with high-grade NETs.
Keywords: Neuroendocrine tumor; PD-L1; RB1; TP53; lung; next-generation sequencing.
Copyright© 2020, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
The Authors declare no conflicts of interest.
Figures




References
-
- Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, Geisinger K, Hirsch FR, Ishikawa Y, Kerr KM, Noguchi M, Pelosi G, Powell CA, Tsao MS, Wistuba I, WHO Panel The 2015 world health organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–1260. doi: 10.1097/JTO.0000000000000630. - DOI - PubMed
-
- Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, Busam KJ, de Krijger RR, Dietel M, El-Naggar AK, Fernandez-Cuesta L, Kloppel G, McCluggage WG, Moch H, Ohgaki H, Rakha EA, Reed NS, Rous BA, Sasano H, Scarpa A, Scoazec JY, Travis WD, Tallini G, Trouillas J, van Krieken JH, Cree IA. A common classification framework for neuroendocrine neoplasms: An international agency for research on cancer (iarc) and world health organization (who) expert consensus proposal. Mod Pathol. 2018;31(12):1770–1786. doi: 10.1038/s41379-018-0110-y. - DOI - PMC - PubMed
-
- Alcala N, Leblay N, Gabriel AAG, Mangiante L, Hervas D, Giffon T, Sertier AS, Ferrari A, Derks J, Ghantous A, Delhomme TM, Chabrier A, Cuenin C, Abedi-Ardekani B, Boland A, Olaso R, Meyer V, Altmuller J, Le Calvez-Kelm F, Durand G, Voegele C, Boyault S, Moonen L, Lemaitre N, Lorimier P, Toffart AC, Soltermann A, Clement JH, Saenger J, Field JK, Brevet M, Blanc-Fournier C, Galateau-Salle F, Le Stang N, Russell PA, Wright G, Sozzi G, Pastorino U, Lacomme S, Vignaud JM, Hofman V, Hofman P, Brustugun OT, Lund-Iversen M, Thomas de Montpreville V, Muscarella LA, Graziano P, Popper H, Stojsic J, Deleuze JF, Herceg Z, Viari A, Nuernberg P, Pelosi G, Dingemans AMC, Milione M, Roz L, Brcic L, Volante M, Papotti MG, Caux C, Sandoval J, Hernandez-Vargas H, Brambilla E, Speel EJM, Girard N, Lantuejoul S, McKay JD, Foll M, Fernandez-Cuesta L. Integrative and comparative genomic analyses identify clinically relevant pulmonary carcinoid groups and unveil the supra-carcinoids. Nat Commun. 2019;10(1):3407. doi: 10.1038/s41467-019-11276-9. - DOI - PMC - PubMed
-
- George J, Walter V, Peifer M, Alexandrov LB, Seidel D, Leenders F, Maas L, Muller C, Dahmen I, Delhomme TM, Ardin M, Leblay N, Byrnes G, Sun R, De Reynies A, McLeer-Florin A, Bosco G, Malchers F, Menon R, Altmuller J, Becker C, Nurnberg P, Achter V, Lang U, Schneider PM, Bogus M, Soloway MG, Wilkerson MD, Cun Y, McKay JD, Moro-Sibilot D, Brambilla CG, Lantuejoul S, Lemaitre N, Soltermann A, Weder W, Tischler V, Brustugun OT, Lund-Iversen M, Helland A, Solberg S, Ansen S, Wright G, Solomon B, Roz L, Pastorino U, Petersen I, Clement JH, Sanger J, Wolf J, Vingron M, Zander T, Perner S, Travis WD, Haas SA, Olivier M, Foll M, Buttner R, Hayes DN, Brambilla E, Fernandez-Cuesta L, Thomas RK. Integrative genomic profiling of large-cell neuroendocrine carcinomas reveals distinct subtypes of high-grade neuroendocrine lung tumors. Nat Commun. 2018;9(1):1048. doi: 10.1038/s41467-018-03099-x. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
