Comparison of the Outcome of Patients Protected by the Wearable Cardioverter Defibrillator (WCD) for <90 Wear Days versus ≥90 Wear Days
- PMID: 33144474
- PMCID: PMC7811610
- DOI: 10.21873/invivo.12205
Comparison of the Outcome of Patients Protected by the Wearable Cardioverter Defibrillator (WCD) for <90 Wear Days versus ≥90 Wear Days
Abstract
Background/aim: The wearable cardioverter/defibrillator (WCD) is recommended to prevent sudden cardiac death (SCD). Guidelines suggest a 90 days' period, but prolongation of WCD wear time until increasing the ejection fraction (≥35%) might be suggested.
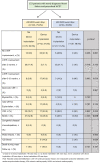
Patients and methods: A cohort of 153 patients with prescribed WCD were divided into two groups: A <90 wear days' group (n=112) vs. ≥90 wear days' group (n=41) and followed.
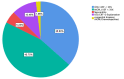
Results: In the first group, WCD shock occurred in 3.6% of patients, 47.3% improved in left ventricular ejection fraction (LVEF) after 3 months, and 37.5% had a cardiac implantable electronic device (CIED) implantation with appropriate implantable cardioverter defibrillator (ICD) shock events occurring in 6 patients. Two of these patients already received WCD shock therapy due to ventricular fibrillation. A 20.5% improved in LVEF after 6-12 months, but 73% were already implanted with ICD. In the second group, 4.9% received WCD shock, 34.1% improved in LVEF after 3 months, 48.8% were implanted with ICD, and 2 had ICD shocks during follow up time. LVEF improvement after 6-12 months occurred in 26.8%. ICD implantation was prevented in 7.3% of patients due to LVEF recovery.
Conclusion: Prolonging wearing days of WCD may reduce the number of inappropriate ICD implantation.
Keywords: Wearable cardioverter defibrillator (WCD); heart insufficiency; sudden cardiac death.
Copyright© 2020, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
All Authors declare that there are no conflicts of interest regarding this study.
Figures
References
-
- Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekval TM, Spaulding C, Van Veldhuisen DJ, Group ESCSD. 2015 esc guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the european society of cardiology (esc). Endorsed by: Association for european paediatric and congenital cardiology (aepc) Eur Heart J. 2015;36(41):2793–2867. doi: 10.1093/eurheartj/ehv316. - DOI - PubMed
-
- Piccini JP Sr., Allen LA, Kudenchuk PJ, Page RL, Patel MR, Turakhia MP, American Heart Association Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing Wearable cardioverter-defibrillator therapy for the prevention of sudden cardiac death: A science advisory from the american heart association. Circulation. 2016;133(17):1715–1727. doi: 10.1161/CIR.0000000000000394. - DOI - PubMed
-
- Brooks GC, Lee BK, Rao R, Lin F, Morin DP, Zweibel SL, Buxton AE, Pletcher MJ, Vittinghoff E, Olgin JE, PREDICTS Investigators Predicting persistent left ventricular dysfunction following myocardial infarction: The predicts study. J Am Coll Cardiol. 2016;67(10):1186–1196. doi: 10.1016/j.jacc.2015.12.042. - DOI - PMC - PubMed
-
- Duncker D, Konig T, Hohmann S, Bauersachs J, Veltmann C. Avoiding untimely implantable cardioverter/defibrillator implantation by intensified heart failure therapy optimization supported by the wearable cardioverter/defibrillator-the prolong study. J Am Heart Assoc. 2017;6(1) doi: 10.1161/JAHA.116.004512. - DOI - PMC - PubMed
-
- Kutyifa V, Moss AJ, Klein H, Biton Y, McNitt S, MacKecknie B, Zareba W, Goldenberg I. Use of the wearable cardioverter defibrillator in high-risk cardiac patients: Data from the prospective registry of patients using the wearable cardioverter defibrillator (wearit-ii registry) Circulation. 2015;132(17):1613–1619. doi: 10.1161/CIRCULATIONAHA.115.015677. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources