Identification and elimination of false positives in electrochemical nitrogen reduction studies
- PMID: 33144566
- PMCID: PMC7641139
- DOI: 10.1038/s41467-020-19130-z
Identification and elimination of false positives in electrochemical nitrogen reduction studies
Abstract
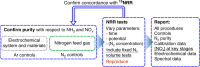
Ammonia is of emerging interest as a liquefied, renewable-energy-sourced energy carrier for global use in the future. Electrochemical reduction of N2 (NRR) is widely recognised as an alternative to the traditional Haber-Bosch production process for ammonia. However, though the challenges of NRR experiments have become better understood, the reported rates are often too low to be convincing that reduction of the highly unreactive N2 molecule has actually been achieved. This perspective critically reassesses a wide range of the NRR reports, describes experimental case studies of potential origins of false-positives, and presents an updated, simplified experimental protocol dealing with the recently emerging issues.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- MacFarlane DR, et al. A roadmap to the ammonia economy. Joule. 2020;4:1186–1205. doi: 10.1016/j.joule.2020.04.004. - DOI
-
- Choi, J. et al. Promoting nitrogen electroreduction to ammonia with bismuth nanocrystals and potassium cations in water. Preprint at 10.26434/chemrxiv.11768814.v11768811 (2020).
-
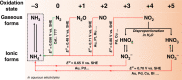
- Choi J, et al. Electroreduction of nitrates, nitrites and gaseous nitrogen oxides: a potential source of ammonia in dinitrogen reduction (NRR) studies. ACS Energy Lett. 2020;5:2095–2097. doi: 10.1021/acsenergylett.0c00924. - DOI
-
- Giddey S, Badwal S, Kulkarni A. Review of electrochemical ammonia production technologies and materials. Int. J. Hydrog. Energy. 2013;38:14576–14594. doi: 10.1016/j.ijhydene.2013.09.054. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

