Concomitant genetic ablation of L-type Cav1.3 (α1D) and T-type Cav3.1 (α1G) Ca2+ channels disrupts heart automaticity
- PMID: 33144668
- PMCID: PMC7642305
- DOI: 10.1038/s41598-020-76049-7
Concomitant genetic ablation of L-type Cav1.3 (α1D) and T-type Cav3.1 (α1G) Ca2+ channels disrupts heart automaticity
Abstract
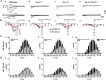
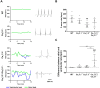
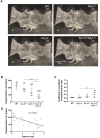
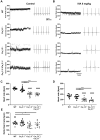
Cardiac automaticity is set by pacemaker activity of the sinus node (SAN). In addition to the ubiquitously expressed cardiac voltage-gated L-type Cav1.2 Ca2+ channel isoform, pacemaker cells within the SAN and the atrioventricular node co-express voltage-gated L-type Cav1.3 and T-type Cav3.1 Ca2+ channels (SAN-VGCCs). The role of SAN-VGCCs in automaticity is incompletely understood. We used knockout mice carrying individual genetic ablation of Cav1.3 (Cav1.3-/-) or Cav3.1 (Cav3.1-/-) channels and double mutant Cav1.3-/-/Cav3.1-/- mice expressing only Cav1.2 channels. We show that concomitant loss of SAN-VGCCs prevents physiological SAN automaticity, blocks impulse conduction and compromises ventricular rhythmicity. Coexpression of SAN-VGCCs is necessary for impulse formation in the central SAN. In mice lacking SAN-VGCCs, residual pacemaker activity is predominantly generated in peripheral nodal and extranodal sites by f-channels and TTX-sensitive Na+ channels. In beating SAN cells, ablation of SAN-VGCCs disrupted late diastolic local intracellular Ca2+ release, which demonstrates an important role for these channels in supporting the sarcoplasmic reticulum based "Ca2+ clock" mechanism during normal pacemaking. These data implicate an underappreciated role for co-expression of SAN-VGCCs in heart automaticity and define an integral role for these channels in mechanisms that control the heartbeat.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

