Antibiofilm activity of flavonoids on staphylococcal biofilms through targeting BAP amyloids
- PMID: 33144670
- PMCID: PMC7641273
- DOI: 10.1038/s41598-020-75929-2
Antibiofilm activity of flavonoids on staphylococcal biofilms through targeting BAP amyloids
Abstract
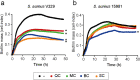
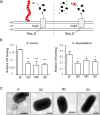
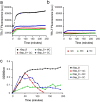
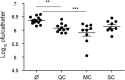
The opportunistic pathogen Staphylococcus aureus is responsible for causing infections related to indwelling medical devices, where this pathogen is able to attach and form biofilms. The intrinsic properties given by the self-produced extracellular biofilm matrix confer high resistance to antibiotics, triggering infections difficult to treat. Therefore, novel antibiofilm strategies targeting matrix components are urgently needed. The Biofilm Associated Protein, Bap, expressed by staphylococcal species adopts functional amyloid-like structures as scaffolds of the biofilm matrix. In this work we have focused on identifying agents targeting Bap-related amyloid-like aggregates as a strategy to combat S. aureus biofilm-related infections. We identified that the flavonoids, quercetin, myricetin and scutellarein specifically inhibited Bap-mediated biofilm formation of S. aureus and other staphylococcal species. By using in vitro aggregation assays and the cell-based methodology for generation of amyloid aggregates based on the Curli-Dependent Amyloid Generator system (C-DAG), we demonstrated that these polyphenols prevented the assembly of Bap-related amyloid-like structures. Finally, using an in vivo catheter infection model, we showed that quercetin and myricetin significantly reduced catheter colonization by S. aureus. These results support the use of polyphenols as anti-amyloids molecules that can be used to treat biofilm-related infections.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Common plant flavonoids prevent the assembly of amyloid curli fibres and can interfere with bacterial biofilm formation.Environ Microbiol. 2020 Dec;22(12):5280-5299. doi: 10.1111/1462-2920.15216. Epub 2020 Sep 22. Environ Microbiol. 2020. PMID: 32869465
-
Bacterial biofilm functionalization through Bap amyloid engineering.NPJ Biofilms Microbiomes. 2022 Aug 1;8(1):62. doi: 10.1038/s41522-022-00324-w. NPJ Biofilms Microbiomes. 2022. PMID: 35909185 Free PMC article.
-
Small Molecules Produced by Commensal Staphylococcus epidermidis Disrupt Formation of Biofilms by Staphylococcus aureus.Appl Environ Microbiol. 2020 Feb 18;86(5):e02539-19. doi: 10.1128/AEM.02539-19. Print 2020 Feb 18. Appl Environ Microbiol. 2020. PMID: 31862721 Free PMC article.
-
Innovative approaches to treat Staphylococcus aureus biofilm-related infections.Essays Biochem. 2017 Mar 3;61(1):61-70. doi: 10.1042/EBC20160056. Print 2017 Feb 28. Essays Biochem. 2017. PMID: 28258230 Review.
-
Bacterial functional amyloids: Order from disorder.Biochim Biophys Acta Proteins Proteom. 2019 Oct;1867(10):954-960. doi: 10.1016/j.bbapap.2019.05.010. Epub 2019 Jun 10. Biochim Biophys Acta Proteins Proteom. 2019. PMID: 31195143 Free PMC article. Review.
Cited by
-
Macro- and Microelement Composition, Antioxidant Activity, and Biological Effect of Cold-Pressed Edible Oils from Commercial and Amateur Companies.Molecules. 2025 Mar 23;30(7):1425. doi: 10.3390/molecules30071425. Molecules. 2025. PMID: 40286008 Free PMC article.
-
Antibiofilm activity of Plumbagin against Staphylococcus aureus.Sci Rep. 2025 Mar 7;15(1):7948. doi: 10.1038/s41598-025-92435-5. Sci Rep. 2025. PMID: 40055436 Free PMC article.
-
Antibiofilm and Antivirulence Potentials of 3,2'-Dihydroxyflavone against Staphylococcus aureus.Int J Mol Sci. 2024 Jul 24;25(15):8059. doi: 10.3390/ijms25158059. Int J Mol Sci. 2024. PMID: 39125628 Free PMC article.
-
Inhibition of Biofilm Formation by the Synergistic Action of EGCG-S and Antibiotics.Antibiotics (Basel). 2021 Jan 21;10(2):102. doi: 10.3390/antibiotics10020102. Antibiotics (Basel). 2021. PMID: 33494273 Free PMC article.
-
Strategies to prevent, curb and eliminate biofilm formation based on the characteristics of various periods in one biofilm life cycle.Front Cell Infect Microbiol. 2022 Sep 21;12:1003033. doi: 10.3389/fcimb.2022.1003033. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36211965 Free PMC article. Review.
References
-
- Sklyar TV, Lavrentieva KV, Alyonkina YA, Kolomoets AM, Vinnikov AI. Resistance of nosocomial strains to antibacterial drugs and its link to biofilm formation. Regul. Mech. Biosyst. 2018;8:540–546. doi: 10.15421/021783. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

