Tumour treating fields therapy for glioblastoma: current advances and future directions
- PMID: 33144698
- PMCID: PMC7884384
- DOI: 10.1038/s41416-020-01136-5
Tumour treating fields therapy for glioblastoma: current advances and future directions
Erratum in
-
Correction: Tumour treating fields therapy for glioblastoma: current advances and future directions.Br J Cancer. 2021 Aug;125(4):623. doi: 10.1038/s41416-021-01451-5. Br J Cancer. 2021. PMID: 34112950 Free PMC article. No abstract available.
Abstract
Glioblastoma multiforme (GBM) is the most common primary brain tumour in adults and continues to portend poor survival, despite multimodal treatment using surgery and chemoradiotherapy. The addition of tumour-treating fields (TTFields)-an approach in which alternating electrical fields exert biophysical force on charged and polarisable molecules known as dipoles-to standard therapy, has been shown to extend survival for patients with newly diagnosed GBM, recurrent GBM and mesothelioma, leading to the clinical approval of this approach by the FDA. TTFields represent a non-invasive anticancer modality consisting of low-intensity (1-3 V/cm), intermediate-frequency (100-300 kHz), alternating electric fields delivered via cutaneous transducer arrays configured to provide optimal tumour-site coverage. Although TTFields were initially demonstrated to inhibit cancer cell proliferation by interfering with mitotic apparatus, it is becoming increasingly clear that TTFields show a broad mechanism of action by disrupting a multitude of biological processes, including DNA repair, cell permeability and immunological responses, to elicit therapeutic effects. This review describes advances in our current understanding of the mechanisms by which TTFields mediate anticancer effects. Additionally, we summarise the landscape of TTFields clinical trials across various cancers and consider how emerging preclinical data might inform future clinical applications for TTFields.
Conflict of interest statement
O.R. and S.p.J.C. are recipients of an Inovitro™ system (on loan from Novocure) and take part in the annual Inovitro™ Users Meeting hosted by Novocure. The remaining authors declare no competing interests.
Figures


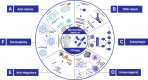
Comment in
-
Insight into the public's interest in tumour treating fields.Br J Cancer. 2021 Sep;125(6):901-903. doi: 10.1038/s41416-021-01504-9. Epub 2021 Jul 27. Br J Cancer. 2021. PMID: 34316021 Free PMC article. No abstract available.
References
-
- Patel, A. P., Fisher, J. L., Nichols, E., Abd-Allah, F., Abdela, J., Abdelalim, A., Abraha, H. N., Agius, D., Alahdab, F., Alam, T. & Allen, C.A. Global, regional, and national burden of brain and other CNS cancer, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18, 376–393 (2019). - PMC - PubMed
-
- Alexander BM, Ba S, Berger MS, Berry DA, Cavenee WK, Chang SM, et al. Adaptive global innovative learning environment for glioblastoma: GBM AGILE. Clin. Cancer Res. 2018;24:737–743. - PubMed
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. - PubMed
Publication types
MeSH terms
Grants and funding
- NC/T001895/1/NC3RS_/National Centre for the Replacement, Refinement and Reduction of Animals in Research/United Kingdom
- 151681/Royal College of Surgeons of England (RCS)
- NC/T002093/1/NC3RS_/National Centre for the Replacement, Refinement and Reduction of Animals in Research/United Kingdom
- STH: 21181; URMS: 164490/Sheffield Hospitals Charity (Sheffield Hospitals Charitable Trust)
- 12102/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

